Exam 24: Nuclear Reactions and Their Applications
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Which one of the following equations correctly represents alpha decay of 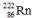 ?
?
Free
(Multiple Choice)
4.7/5  (31)
(31)
Correct Answer:
A
The isotopes of promethium, 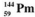 and
and 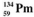 are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
are unstable, and lie on opposite sides of the "line of stability". Which of the following combinations is most likely to represent the type of decay for these isotopes?
Free
(Multiple Choice)
5.0/5  (38)
(38)
Correct Answer:
A
Which one of the following equations correctly represents positron decay of 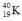 ?
?
Free
(Multiple Choice)
4.7/5  (44)
(44)
Correct Answer:
E
An alkaline earth element is radioactive. It and its daughter elements decay by emitting a total of three alpha particles in succession. In what group of the periodic table is the element resulting from the emission of the third alpha particle?
(Multiple Choice)
4.8/5  (41)
(41)
Cesium-134 is a β emitter with a half-life of 2.0 years. How much of a 2.50-g sample of cesium-134 will remain after 10 years?
(Multiple Choice)
4.9/5  (32)
(32)
Select the nuclide that completes the following nuclear reaction. 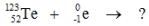
(Multiple Choice)
4.8/5  (33)
(33)
The isotope 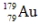 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 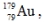 approximately how many such atoms were there present 30 seconds earlier?
approximately how many such atoms were there present 30 seconds earlier?
(Multiple Choice)
4.8/5  (39)
(39)
After 4 half-lives, the fraction of a radioactive isotope which still remains is approximately one-eighth.
(True/False)
4.8/5  (35)
(35)
All the disintegrations of a sample of an unknown nuclide weighing 4.6 × 10-2 g were counted. In the first half-life of the sample, the total number of disintegrations counted was 4.3 × 1020. What is the atomic weight of the unknown element?
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following isotopes is most likely to be unstable?
(Multiple Choice)
4.8/5  (29)
(29)
A certain isotope has a specific activity of 7.29 × 10-4 Ci/g. How many α particles will a 75.0 mg sample emit in one hour?
(Multiple Choice)
4.9/5  (38)
(38)
Select the nuclide that completes the following nuclear reaction. 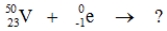
(Multiple Choice)
4.9/5  (37)
(37)
The s-process involves a slow succession of neutron absorption and beta decay processes during the normal life of a star.
(True/False)
4.7/5  (39)
(39)
Which of the following isotopes is most likely to be unstable?
(Multiple Choice)
4.9/5  (46)
(46)
Select the nuclide that completes the following nuclear reaction. 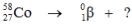
(Multiple Choice)
4.8/5  (32)
(32)
Showing 1 - 20 of 81
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)