Exam 10: The Shapes of Molecules
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
Use VSEPR theory to decide which one of the following ions and molecules is likely to be planar. (The central atom is always first in the formula.)
(Multiple Choice)
4.9/5  (38)
(38)
According to VSEPR theory, a molecule with the general formula AX5 will have a ______ molecular shape.
(Multiple Choice)
4.9/5  (36)
(36)
What is the molecular shape of ClF4- as predicted by the VSEPR theory?
(Multiple Choice)
4.9/5  (32)
(32)
Predict the ideal bond angles in FNO using the molecular shape given by the VSEPR theory.
(Multiple Choice)
4.8/5  (42)
(42)
Predict the ideal bond angles in AsCl3 using the molecular shape given by the VSEPR theory.
(Multiple Choice)
4.8/5  (35)
(35)
Use VSEPR theory to decide which one of the following molecules and ions will have a trigonal pyramidal geometry. (The central atom is always first in the formula.)
(Multiple Choice)
4.9/5  (50)
(50)
All possible resonance structures contribute equally to the resonance hybrid.
(True/False)
4.8/5  (42)
(42)
In which of the following does the nitrogen atom have a formal charge of -1?
(Multiple Choice)
4.9/5  (39)
(39)
In which one of the following species is the central atom (the first atom in the formula) likely to violate the octet rule?
(Multiple Choice)
4.8/5  (43)
(43)
Phosphoryl iodide is used in the preparation of organophosphorus derivatives and phosphate esters. Select the Lewis structure for POI3 that minimizes formal charges.
(Multiple Choice)
4.9/5  (42)
(42)
According to VSEPR theory, a molecule with the general formula AX3 will have a ______ molecular shape.
(Multiple Choice)
4.8/5  (40)
(40)
What is the molecular shape of N2O as predicted by the VSEPR theory? 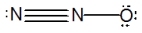
(Multiple Choice)
4.9/5  (47)
(47)
What is the molecular shape of SCl3F as predicted by the VSEPR theory?
(Multiple Choice)
5.0/5  (31)
(31)
What is the molecular shape of the thiocyanate anion, SCN-, as predicted by the VSEPR theory? (Carbon is the central atom.)
(Multiple Choice)
4.9/5  (35)
(35)
According to VSEPR theory, a molecule with the general formula AX3E will have a _____ molecular shape.
(Multiple Choice)
4.8/5  (39)
(39)
What is the molecular shape of ClF2- as predicted by the VSEPR theory?
(Multiple Choice)
4.8/5  (36)
(36)
Which one of the following Lewis structures is definitely incorrect?
(Multiple Choice)
4.8/5  (36)
(36)
Predict the ideal bond angles in GeCl4 using the molecular shape given by the VSEPR theory.
(Multiple Choice)
4.9/5  (28)
(28)
According to VSEPR theory, a molecule with the general formula AX2E will have a ______ molecular shape.
(Multiple Choice)
5.0/5  (42)
(42)
Showing 61 - 80 of 98
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)