Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving
Exam 1: Keys to Studying Chemistry Definitions, Units, and Problem Solving71 Questions
Exam 2: The Components of Matter100 Questions
Exam 3: Stoichiometry of Formulas and Equations70 Questions
Exam 4: Three Major Classes of Chemical Reactions111 Questions
Exam 5: Gases and the Kinetic-Molecular Theory97 Questions
Exam 6: Thermochemistry Energy Flow and Chemical Change72 Questions
Exam 7: Quantum Theory and Atomic Structure69 Questions
Exam 8: Electron Configuration and Chemical Periodicity77 Questions
Exam 9: Models of Chemical Bonding61 Questions
Exam 10: The Shapes of Molecules98 Questions
Exam 11: Theories of Covalent Bonding48 Questions
Exam 12: Intermolecular Forces Liquids, Solids, and Phase Changes90 Questions
Exam 13: The Properties of Mixtures Solutions and Colloids96 Questions
Exam 14: Periodic Patterns in the Main-Group Elements102 Questions
Exam 15: Organic Compounds and the Atomic Properties of Carbon107 Questions
Exam 16: Kinetics Rates and Mechanisms of Chemical Reactions78 Questions
Exam 17: Equilibrium the Extent of Chemical Reactions97 Questions
Exam 18: Acid-Base Equilibria100 Questions
Exam 19: Ionic Equilibria in Aqueous Systems114 Questions
Exam 20: Thermodynamics Entropy, Free Energy, and Reaction Direction84 Questions
Exam 21: Electrochemistry Chemical Change and Electrical Work100 Questions
Exam 22: The Elements in Nature and Industry45 Questions
Exam 23: Transition Elements and Their Coordination Compounds82 Questions
Exam 24: Nuclear Reactions and Their Applications81 Questions
Select questions type
The appropriate number of significant figures in the result of 15.234 × 15.208 is
(Multiple Choice)
4.9/5  (27)
(27)
A scientist made careful measurements of the pressure and temperature of many different gases. Based on these measurements, he concluded that "the pressure of a fixed amount of gas, measured at constant volume, is directly proportional to its absolute temperature." This statement is best described as a
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following correctly expresses 52,030.2 m in scientific notation?
(Multiple Choice)
4.8/5  (42)
(42)
A student makes several measurements of the density of an unknown mineral sample. She then reports the average value of these measurements. The number of significant figures she uses in her result should be a measure of its
(Multiple Choice)
4.7/5  (25)
(25)
Select the answer that expresses the result of this calculation with the correct number of significant figures. 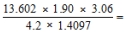
(Multiple Choice)
4.9/5  (37)
(37)
At a pressure of one billionth (10-9) of atmospheric pressure, there are about 2.7 × 1010 molecules in one cubic centimeter of a gas. How many molecules is this per cubic meter?
(Multiple Choice)
4.8/5  (31)
(31)
The speed needed to escape the pull of Earth's gravity is 11.3 km/s. What is this speed in mi/h?
(Multiple Choice)
4.8/5  (36)
(36)
During the swing of a frictionless pendulum, what energy form(s) remain constant?
(Multiple Choice)
4.7/5  (29)
(29)
A large pizza has a diameter of 15 inches. Express this diameter in centimeters.
(Multiple Choice)
4.9/5  (35)
(35)
The distance between carbon atoms in ethylene is 134 picometers. Which of the following expresses that distance in meters?
(Multiple Choice)
4.8/5  (34)
(34)
The ripening of fruit, once picked, is an example of physical change.
(True/False)
5.0/5  (33)
(33)
The density of mercury, the only metal to exist as a liquid at room temperature, is 13.6 g/cm3. What is that density in pounds per cubic inch?
(Multiple Choice)
4.7/5  (31)
(31)
In an average year, the American chemical industry produces more than 9.5 million metric tons of sodium carbonate. Over half of this is used in the manufacture of glass while another third is used in the production of detergents and other chemicals. How many pounds of sodium carbonate are produced annually?
(Multiple Choice)
4.8/5  (35)
(35)
If the price of gold at the morning fixing in London was $5310 per lb, what would a kilogram of gold have cost in £ (pounds)? (Assume an exchange rate of $1.00 = £0.545)
(Multiple Choice)
4.9/5  (33)
(33)
Acetic acid boils at 244.2°F. What is its boiling point in degrees Celsius?
(Multiple Choice)
4.8/5  (29)
(29)
Which of the following abbreviations of the given SI base unit is incorrect?
(Multiple Choice)
4.8/5  (44)
(44)
Showing 41 - 60 of 71
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)