Exam 2: Atoms, Molecules, and Ions
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Which pair of atoms constitutes a pair of isotopes of the same element?
(Multiple Choice)
5.0/5  (46)
(46)
The formula for the compound formed between aluminum ions and phosphate ions is .
(Multiple Choice)
4.9/5  (33)
(33)
An element in the upper right corner of the periodic table .
(Multiple Choice)
4.8/5  (40)
(40)
The element X has three naturally occurring isotopes. The isotopic masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is
Amu) 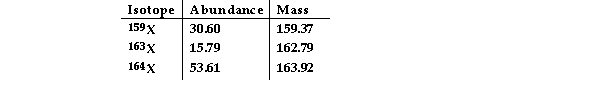
(Multiple Choice)
4.9/5  (35)
(35)
An ion has 8 protons, 9 neutrons, and 10 electrons. The symbol for the ion is .
(Multiple Choice)
4.8/5  (44)
(44)
Which one of the following polyatomic ions has the same charge as the hydroxide ion?
(Multiple Choice)
4.7/5  (42)
(42)
Showing 181 - 200 of 201
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)