Exam 2: Atoms, Molecules, and Ions
Exam 1: Introduction: Matter and Measurement118 Questions
Exam 2: Atoms, Molecules, and Ions201 Questions
Exam 3: Stoichiometry: Calculations With Chemical Formulas and Equations134 Questions
Exam 4: Aqueous Reactions and Solution Stoichiometry147 Questions
Exam 6: Electronic Structure of Atoms161 Questions
Exam 7: Periodic Properties of the Elements149 Questions
Exam 8: Basic Concepts of Chemical Bonding116 Questions
Exam 10: Gases146 Questions
Exam 11: Intermolecular Forces, Liquids, and Solids103 Questions
Exam 12: Modern Materials47 Questions
Exam 13: Properties of Solutions121 Questions
Exam 14: Chemical Kinetics110 Questions
Exam 15: Chemical Equilibrium58 Questions
Exam 16: Acid-Base Equilibria97 Questions
Exam 17: Additional Aspects of Equilibria88 Questions
Exam 18: Chemistry of the Environment105 Questions
Exam 19: Chemical Thermodynamics101 Questions
Exam 20: Electrochemistry90 Questions
Exam 21: Nuclear Chemistry128 Questions
Exam 22: Chemistry of the Nonmetals176 Questions
Exam 23: Metals and Metallurgy112 Questions
Exam 24: Chemistry of Coordination Compounds124 Questions
Exam 25: The Chemistry of Life: Organic and Biological Chemistry115 Questions
Select questions type
Silver has two naturally occurring isotopes with the following isotopic masses:
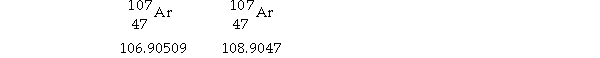 The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is .
The average atomic mass of silver is 107.8682 amu. The fractional abundance of the lighter of the two isotopes is .
(Multiple Choice)
4.9/5  (35)
(35)
Which one of the following is not true concerning cathode rays?
(Multiple Choice)
4.9/5  (26)
(26)
Gravitational forces act between objects in proportion to their
(Multiple Choice)
4.9/5  (33)
(33)
The element X has three naturally occurring isotopes. The masses (amu) and % abundances of the isotopes are given in the table below. The average atomic mass of the element is amu. 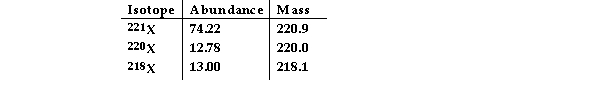
(Multiple Choice)
4.9/5  (30)
(30)
Which pair of substances could be used to illustrate the law of multiple proportions?
(Multiple Choice)
4.8/5  (36)
(36)
What is the formula of the compound formed between strontium ions and nitrogen ions?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 21 - 40 of 201
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)