Exam 7: Changes of State and Gas Laws
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
In which of the following statements is the gas variable (in italics)correctly described?
(Multiple Choice)
4.9/5  (41)
(41)
Henry's law is 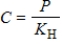 .Which statement BEST describes the meaning of this law?
.Which statement BEST describes the meaning of this law?
(Multiple Choice)
4.9/5  (33)
(33)
Which of the following processes is an endothermic physical change?
(Multiple Choice)
4.9/5  (30)
(30)
Air is primarily composed of nitrogen (594 torr)and oxygen (160 torr).There is also carbon dioxide and water vapor in the air.Assuming that atmospheric pressure is 760 torr, what is the partial pressure of carbon dioxide and water vapor combined?
(Multiple Choice)
4.9/5  (41)
(41)
One of the symptoms of the bends is joint pain.Why does joint pain occur with the bends?
(Multiple Choice)
4.8/5  (37)
(37)
Which statement BEST describes how heat energy is involved in changing water into steam?
(Multiple Choice)
4.8/5  (41)
(41)
Water has a specific heat of 1.00 cal/g·°C, and wood has a specific heat of 0.10 cal/g·°C.Which substance requires more heat to be warmed from room temperature to 50 °C?
(Multiple Choice)
4.8/5  (41)
(41)
This diagram shows balloons of various sizes filled with helium gas.Balloon A is at STP.If you heat balloon A, which balloon would you predict would BEST represent the new size of balloon A? 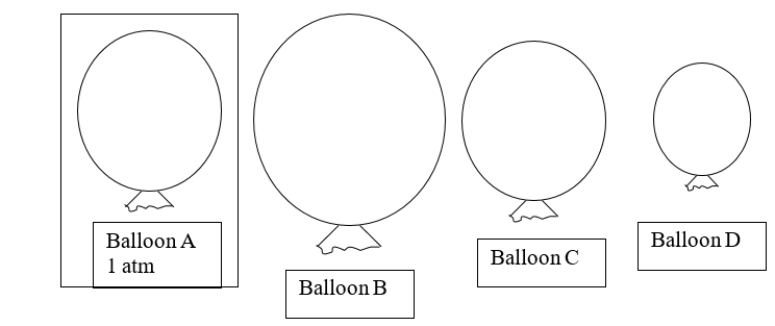
(Multiple Choice)
4.9/5  (37)
(37)
Three boxes, each containing molecules in the gas phase, are illustrated below.Which box would you expect to have the highest pressure? 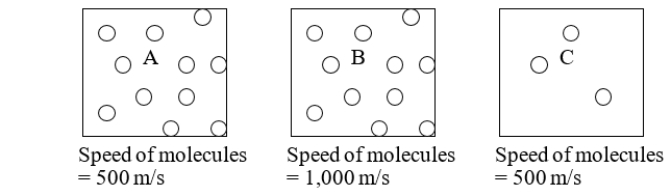
(Multiple Choice)
4.7/5  (42)
(42)
The total pressure in a mixture of gases is equal to the partial pressure(s)of
(Multiple Choice)
4.8/5  (38)
(38)
What is the electron geometry of the carbon indicated by the arrow in this molecule of acetone? 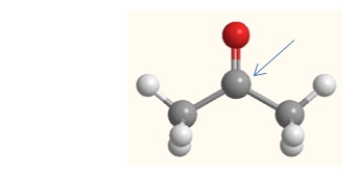
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following physical changes is the result of a decrease in temperature?
(Multiple Choice)
4.8/5  (42)
(42)
You drive your car from Salt Lake City to the mountains to go skiing.It is a nice day in Salt Lake, with a temperature of 16 °C and an atmospheric pressure of 0.85 atm.In the mountains, it is freezing at -1.0 °C and an atmospheric pressure of 0.70 atm.If the volume of air in your tires is 12 L when you leave the city, what is it in the mountains?
(Multiple Choice)
4.9/5  (39)
(39)
What is the strongest intermolecular force that exists between molecules of acetone in the liquid or solid state? 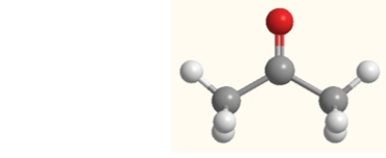
(Multiple Choice)
4.8/5  (36)
(36)
Which process requires more energy per gram: melting ice or boiling water?
(Multiple Choice)
4.8/5  (30)
(30)
What happens to the speed of molecules when the molecules are frozen?
(Multiple Choice)
4.8/5  (39)
(39)
Showing 81 - 99 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)