Exam 7: Changes of State and Gas Laws
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Acetone, shown here, is a common solvent and component of fingernail polish remover.Which of the following Lewis structures is acetone? 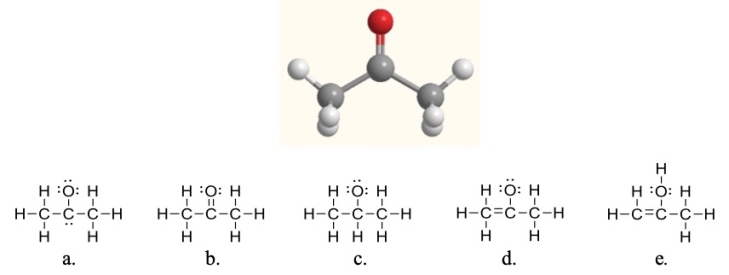
(Multiple Choice)
4.8/5  (35)
(35)
At 1.0 atm, the air in a pilot's lungs occupies 0.50 L.The pilot ascends to an altitude where the pressure is 0.35 atm.If the pilot holds her breath during her ascent, what is the new volume of the air in the pilot's lungs?
(Multiple Choice)
4.8/5  (36)
(36)
Which statement about vaporization and evaporation is FALSE?
(Multiple Choice)
4.9/5  (34)
(34)
According to Avogadro's law, 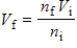 .What is the meaning of this law?
.What is the meaning of this law?
(Multiple Choice)
4.7/5  (33)
(33)
Balloon A is placed into a container that has a higher pressure than atmospheric pressure.Which of the following statements about the relationship between pressure and volume is TRUE?
(Multiple Choice)
4.8/5  (45)
(45)
A scuba diver dives down to 15 m, where the pressure is 2.5 atm.The scuba diver then inhales 500.mL of air and holds his breath while ascending to the water surface, where the pressure is 1 atm.What is the volume of the air in the diver's lungs at the surface? Assume that T and n are constant.
(Multiple Choice)
4.9/5  (31)
(31)
Why is water (H2O)a liquid at room temperature whereas methane (CH4)is a gas?
(Multiple Choice)
4.9/5  (29)
(29)
Which statement BEST describes how the kinetic molecular view of gases can be used to explain the effect of gas temperature on gas pressure?
(Multiple Choice)
4.8/5  (27)
(27)
The two beakers below each have added to them the same amount of heat energy.Which statement BEST describes what would happen to the temperatures of the two beakers? 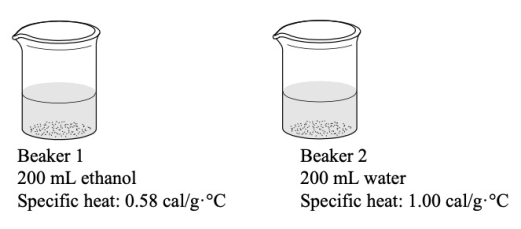
(Multiple Choice)
4.8/5  (30)
(30)
Atmospheric pressure at sea level is ________.At elevations higher than sea level, atmospheric pressure is_________.
(Multiple Choice)
4.8/5  (36)
(36)
At the grocery store, a bag of chips contains 0.10 L of air at 1.0 atm and 20 °C.The unopened bag of chips is taken on a camping trip in the mountains where the pressure is 0.90 atm and the temperature is 25 °C.What is the volume of air in the bag in the mountains?
(Multiple Choice)
4.9/5  (42)
(42)
Which bond in acetone, shown below, is correctly labeled with a dipole arrow? 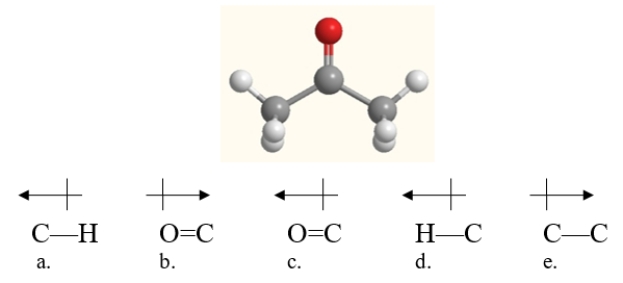
(Multiple Choice)
4.8/5  (31)
(31)
To accomplish the change of phase shown in the diagram below, you could 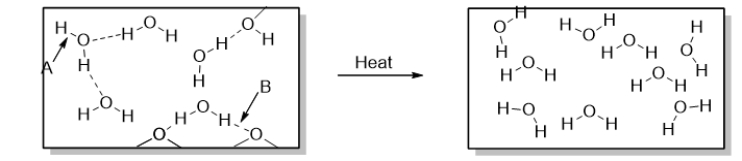
(Multiple Choice)
5.0/5  (43)
(43)
Which of the following changes is NOT correctly labeled as a chemical reaction or a change of state?
(Multiple Choice)
4.9/5  (46)
(46)
Which statement BEST describes why a higher concentration of carbon dioxide is present in exhaled breath compared to inhaled breath?
(Multiple Choice)
4.8/5  (39)
(39)
A narrow tube on a road bike should be inflated to about 100 psi.What is this pressure in atmospheres?
(Multiple Choice)
5.0/5  (39)
(39)
Imagine that you have a beaker of gas molecules as shown in the image below.If all of the gas in the beaker is transferred to a new beaker half the size of the original beaker, while maintaining the same temperature, which view BEST represents what the gas would look like? 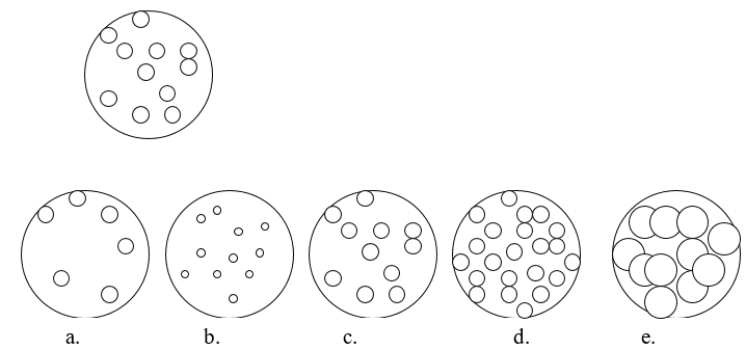
(Multiple Choice)
4.9/5  (34)
(34)
What happens when a large volume of gas is compressed to a smaller volume?
(Multiple Choice)
4.9/5  (35)
(35)
Showing 61 - 80 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)