Exam 7: Changes of State and Gas Laws
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Consider a warm summer's day at the beach.While the sand feels warm on your feet, the water feels cool.Which statement is the BEST explanation for this phenomenon?
(Multiple Choice)
4.7/5  (41)
(41)
Because gases are compressible, they become ________ when pressure is increased.
(Multiple Choice)
5.0/5  (38)
(38)
Which of the following statements BEST describes the process that occurs when air is inhaled into the lungs?
(Multiple Choice)
5.0/5  (41)
(41)
Which has a higher partial pressure of oxygen: inhaled air or exhaled air?
(Multiple Choice)
4.8/5  (42)
(42)
Henry's constant for halothane, an anesthetic, is higher than that of ether.Which anesthetic is faster acting?
(Multiple Choice)
4.7/5  (31)
(31)
You have a balloon containing 1.4 L of gas in it at standard temperature and pressure.Which conversion factor would you use to convert this number to moles?
(Multiple Choice)
4.9/5  (43)
(43)
A gas having a volume of 11.2 L has added to it 0.50 mol of gas at the same temperature and pressure.The final volume is 22.4 L, again at the same temperature and pressure.How many moles were there originally?
(Multiple Choice)
4.8/5  (36)
(36)
Which of the following properties does NOT describe the kinetic-molecular view of gases?
(Multiple Choice)
4.7/5  (37)
(37)
A gas having a volume of 11.2 L has added to it 0.5 mol of gas at the same temperature and pressure.The final volume is 22.4 L, again at the same temperature and pressure.Which relationship is used to determine the number of moles of gas originally?
(Multiple Choice)
4.9/5  (38)
(38)
How many calories of heat are required to raise the temperature of 15 g water (specific heat = 1.00 cal/g·°C)from 25 °C and 42 °C?
(Multiple Choice)
4.9/5  (42)
(42)
On a cool morning (12 °C), a balloon is filled with 1.5 L of helium.By mid-afternoon, the temperature has soared to 32 °C.What is the new volume of the balloon?
(Multiple Choice)
4.8/5  (41)
(41)
What change of phase is represented by B on the heating curve? 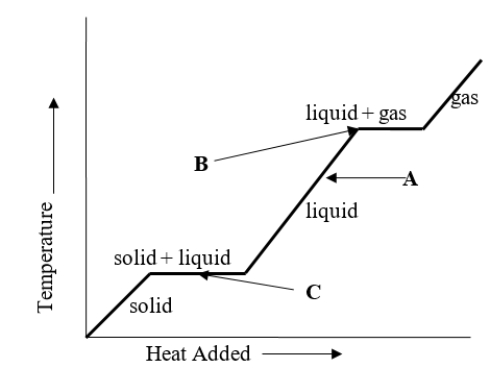
(Multiple Choice)
4.7/5  (41)
(41)
A balloon at STP is heated.Which of the following statements about the relationship between volume and temperature is TRUE?
(Multiple Choice)
4.8/5  (37)
(37)
Which statement BEST describes why steam burns are more severe than boiling water burns?
(Multiple Choice)
4.8/5  (33)
(33)
Balloon A is placed into a container that has a higher pressure than atmospheric pressure.Which balloon would you predict to be the new size of balloon A? 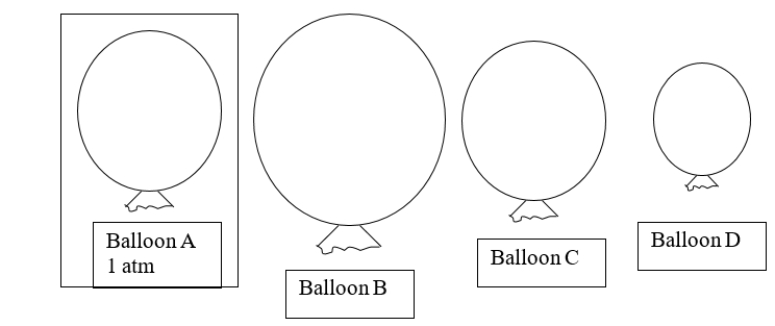
(Multiple Choice)
4.9/5  (42)
(42)
Which of the following statements BEST describes how hyperbaric oxygen therapy is used to treat carbon monoxide poisoning?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following instruments is used to measure atmospheric pressure?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 21 - 40 of 99
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)