Exam 5: Chemical Quantities and Introduction to Reactions
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Which of the following is a consideration in the design of air bags?
(Multiple Choice)
4.8/5  (34)
(34)
The following chemical equation, the decomposition of hydrogen peroxide to water, is not balanced.What must be done to the equation to make it balanced? 2 H2O2(l)→ 2 H2O(l)
(Multiple Choice)
4.9/5  (32)
(32)
Which of the following must occur for there to be a chemical reaction?
(Multiple Choice)
4.8/5  (34)
(34)
Jane Doe has a cholesterol (C27H46O)count of 178 mg/dL.You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood.Which of the following unit conversions are required to calculate the number of cholesterol molecules per deciliter?
i. 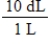 II.
II. 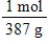 III.
III. 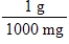 IV.
IV. 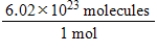
(Multiple Choice)
4.9/5  (31)
(31)
What is the primary relationship between a chemical reaction and a chemical equation?
(Multiple Choice)
4.8/5  (40)
(40)
In an oxidation-reduction reaction, the species that gains electrons is _______.
(Multiple Choice)
4.7/5  (26)
(26)
The balanced equation for the combustion of propane is given below.What do the numbers in front of the molecules (the coefficients)mean? C3H8 + 5 O2 → 3 CO2 + 4 H2O
(Multiple Choice)
4.9/5  (34)
(34)
The balanced chemical equation for the combustion of propane (C3H8)is given below.How many grams of carbon dioxide (CO2)are released when 35 g of propane are burned in the presence of excess oxygen? C3H8 + 5 O2 → 3 CO2 + 4 H2O
(Multiple Choice)
4.9/5  (38)
(38)
How many atoms of oxygen are present in the reactants of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
(Multiple Choice)
4.8/5  (40)
(40)
Is methane (CH4)undergoing oxidation or reduction during this reaction, and how can you tell? CH4 + 2 O2 → CO2 + 2 H2O
(Multiple Choice)
4.9/5  (34)
(34)
A chemical reaction involves many changes but not everything changes during a reaction.Which of the following is NOT a change that occurs during a chemical reaction?
(Multiple Choice)
4.9/5  (33)
(33)
Which statement describes how molecular mass and molar mass differ?
(Multiple Choice)
4.7/5  (34)
(34)
What type of reaction is the addition of hydrochloric acid (HCl)to propene? 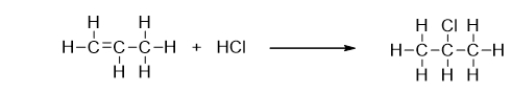
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following is NOT indicated by a chemical equation?
(Multiple Choice)
4.7/5  (25)
(25)
Which of the following correctly determines the formula mass of sodium sulfate (Na2SO4)?
(Multiple Choice)
4.8/5  (25)
(25)
Does one mole of the antibiotic penicillin G (C16H18N2O4S)weigh more or less than one mole of the antibiotic streptomycin (C21H39N7O12)?
(Multiple Choice)
4.8/5  (31)
(31)
Showing 21 - 40 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)