Exam 5: Chemical Quantities and Introduction to Reactions
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
What is the meaning of the arrow in the following chemical equation? 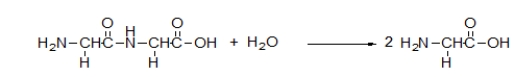
(Multiple Choice)
4.8/5  (39)
(39)
A common, over-the-counter antacid is Al(OH)3.This antacid reacts with gastric juice (HCl)in the stomach, producing AlCl3 and H2O.Which of the following equations can be used to correctly determine how much gastric juice (HCl)reacts with an antacid tablet containing 0.25 grams Al(OH)3? Note that you will need to write a balanced chemical equation to answer this question.
(Multiple Choice)
4.9/5  (35)
(35)
Is this chemical equation balanced? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
(Multiple Choice)
4.8/5  (38)
(38)
Cellular respiration is an example of a(n)___________ reaction.
(Multiple Choice)
4.8/5  (31)
(31)
A molecule of oxygen, O2, has a molar mass of 32.00 g/mol.What is the mass of 4.52 × 1023 O2 molecules?
(Multiple Choice)
4.9/5  (31)
(31)
If Jane Doe has a blood carbon dioxide concentration of 0.022 mol/L, how many carbon dioxide molecules are in each liter of her blood?
(Multiple Choice)
4.9/5  (34)
(34)
What are the products of the reaction illustrated in Figure I? 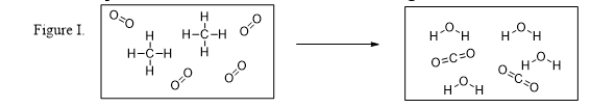
(Multiple Choice)
4.7/5  (31)
(31)
Pentane (C5H12)reacts with oxygen gas (O2)to form carbon dioxide (CO2)and water (H2O)according to the following reaction.What is the coefficient for oxygen in the balanced equation? C5H12 + ?O2 → ?CO2 + ?H2O
(Multiple Choice)
4.9/5  (34)
(34)
Which of the following statements describes the processes that can occur during reduction? I.Loss of electrons
II)Gain of electrons
III)Loss of oxygens
IV)Loss of hydrogens
(Multiple Choice)
4.9/5  (38)
(38)
In the body, glucose is broken down in the presence of oxygen into carbon dioxide and water.The balanced chemical equation for this reaction is shown below.According to this equation, how many molecules of carbon dioxide are produced when three molecules of glucose are metabolized in the presence of 18 moles of oxygen?
C6H12O6 + 6 O2 → 6 CO2 + 6 H2O
(Multiple Choice)
4.9/5  (33)
(33)
Which statement BEST describes the changes that occur over the course of the following oxidation-reduction reaction? Fe + Cu2+ → Fe2+ + Cu
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following is NOT one of the basic types of reactions?
(Multiple Choice)
5.0/5  (41)
(41)
Which statement BEST describes why we use moles when measuring quantities of atoms and molecules?
(Multiple Choice)
4.8/5  (41)
(41)
Which of the following characteristics is NOT shared by all combustion reactions?
(Multiple Choice)
4.8/5  (35)
(35)
Jane Doe has a cholesterol (C27H46O)count of 178 mg/dL.You would like to calculate the number of cholesterol molecules that Jane Doe has in each deciliter of blood.Which of the following calculations is used to solve this problem?
(Multiple Choice)
4.8/5  (30)
(30)
Which of the following equations is used to convert 91.0 milligrams of urea (CH4N2O)to moles of urea?
(Multiple Choice)
4.9/5  (21)
(21)
The following reaction is the formation of a peptide from two amino acids, the sort of reaction that occurs in the body as part of the synthesis of proteins.This reaction is missing a component.Which of the following is missing? 2 NH2CH2COOH → NH2CH2CONHCH2COOH
(Multiple Choice)
4.8/5  (34)
(34)
Showing 61 - 80 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)