Exam 5: Chemical Quantities and Introduction to Reactions
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Calculate the mass of one mole of dopamine, a neurotransmitter with the molecular formula C8H11NO2.
(Multiple Choice)
4.8/5  (39)
(39)
A solution of glucose has 10.00 g glucose (molecular weight = 180.g/mol)in 1.00 L of water.How many molecules of glucose are in 1.00 L of the solution?
(Multiple Choice)
4.8/5  (33)
(33)
When two aqueous solutions of barium chloride (BaCl2)and copper sulfate (CuSO4)are combined, insoluble barium sulfate (BaSO4)forms leaving copper chloride (CuCl2)in solution.What type of reaction is this?
(Multiple Choice)
4.7/5  (27)
(27)
How many atoms of magnesium are present in the reactants of this chemical equation? Mg(OH)2 + 2 HCl → MgCl2 + 2 H2O
(Multiple Choice)
4.9/5  (43)
(43)
Jane Doe has a cholesterol (C27H46O)count of 178 mg/dL.How many cholesterol molecules does Jane Doe have in each deciliter of blood?
(Multiple Choice)
4.7/5  (37)
(37)
Which statement describes how formula mass and molecular mass differ?
(Multiple Choice)
4.8/5  (36)
(36)
The balanced chemical equation for the combustion of butane is given below and is the reaction happening when you use a butane lighter.How many grams of O2 are needed to completely react with 5.0 g of butane? 2 C4H10 + 13 O2 → 8 CO2 + 10 H2O
(Multiple Choice)
4.8/5  (33)
(33)
Coefficients are important components of chemical equations.What is the significance of coefficients in a chemical equation? I.Coefficients indicate the ratio of masses of molecules.
II)Coefficients indicate the ratio of numbers of atoms and molecules.
III)Coefficients indicate the ratio of numbers of moles of molecules and atoms.
(Multiple Choice)
4.7/5  (31)
(31)
How is a nuclear reaction different from a chemical reaction?
(Multiple Choice)
4.8/5  (42)
(42)
Which statement BEST describes the following reaction? Fe + Cu2+ → Fe2+ + Cu
(Multiple Choice)
4.8/5  (40)
(40)
Acetylene (C2H2)is a small organic molecule used in the industrial preparation of many other molecules and also in welding.Acetylene is made from calcium carbide (CaC2)and water and produces acetylene and calcium hydroxide.Which of the following choices is the correct balanced chemical equation for the preparation of acetylene?
(Multiple Choice)
4.8/5  (27)
(27)
Which of the following transformations is a chemical reaction?
(Multiple Choice)
4.8/5  (30)
(30)
What is the balanced chemical equation for the reaction illustrated in Figure I? 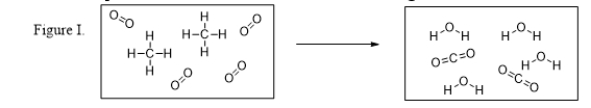
(Multiple Choice)
4.9/5  (40)
(40)
The following reaction is an example of a(n)___________ reaction. CH4 + 2 O2 → CO2 + 2 H2O
I.oxidation-reduction
II.combination
III.combustion
(Multiple Choice)
4.8/5  (32)
(32)
Which of the following is NOT an example of a double displacement reaction?
(Multiple Choice)
4.9/5  (30)
(30)
Showing 41 - 60 of 88
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)