Exam 4: Molecular Geometry, Polarity, and Intermolecular
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Why do electron groups around a central atom arrange themselves as far apart from one another as possible, while still remaining attached to the central atom?
(Multiple Choice)
4.9/5  (38)
(38)
Which of the molecules below exhibits hydrogen bonding forces between like molecules? 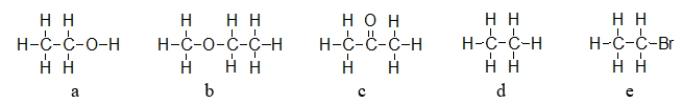
(Multiple Choice)
4.8/5  (35)
(35)
When determining the shape of a molecule, it is necessary to count electron groups.Which of the following is an electron group?
(Multiple Choice)
4.9/5  (46)
(46)
Atom X in a molecule has tetrahedral electron geometry but a bent molecular geometry.Which of the following describes the identity of atom X?
(Multiple Choice)
4.8/5  (39)
(39)
What is the strongest type of intermolecular force of attraction that a caffeine molecule could form with other caffeine molecules? 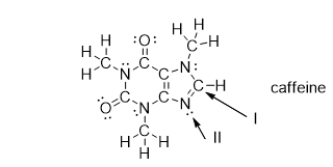
(Multiple Choice)
4.8/5  (36)
(36)
Showing 81 - 86 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)