Exam 4: Molecular Geometry, Polarity, and Intermolecular
Exam 1: Matter, Energy, and Measurement91 Questions
Exam 2: Atomic Structure and Radioisotopes107 Questions
Exam 3: Ionic and Covalent Compounds95 Questions
Exam 4: Molecular Geometry, Polarity, and Intermolecular86 Questions
Exam 5: Chemical Quantities and Introduction to Reactions88 Questions
Exam 6: Chemical Reactions: Energy, Rates, and Equilibrium87 Questions
Exam 7: Changes of State and Gas Laws99 Questions
Exam 8: Mixtures, Solution Concentrations, and Diffusion97 Questions
Exam 9: Acids and Bases, Ph, and Buffers83 Questions
Exam 10: Introduction to Organic Chemistry:111 Questions
Exam 11: Alcohols, Phenols, Thiols, Ethers, and Amines69 Questions
Exam 12: The Carbonyl Containing Functional Groups70 Questions
Exam 13: The Common Organic Reactions in Biochemistry82 Questions
Exam 14: Carbohydrates: Structure and Function97 Questions
Exam 15: Lipids: Structure and Function106 Questions
Exam 16: Proteins: Structure and Function134 Questions
Exam 17: Nucleotides and Nucleic Acids109 Questions
Exam 18: Energy and Metabolism115 Questions
Select questions type
Which figure BEST illustrates how two molecules of ethanol interact? 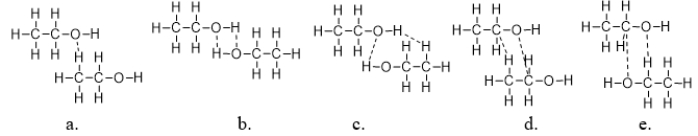
(Multiple Choice)
4.9/5  (30)
(30)
What is the electron geometry of the carbon in formaldehyde, shown below? 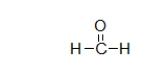
(Multiple Choice)
4.9/5  (38)
(38)
Which molecule below exhibits the strongest intermolecular forces of attraction? 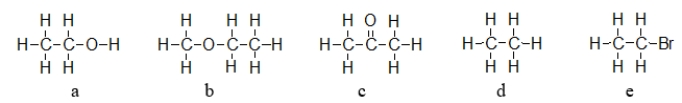
(Multiple Choice)
4.8/5  (33)
(33)
What is the H-C-H bond angle of each carbon in ethylene (C2H4)?
(Multiple Choice)
4.9/5  (39)
(39)
How does VSEPR theory explain the electron group geometry of a molecule?
(Multiple Choice)
4.9/5  (46)
(46)
Electrostatic interactions between positive and negative ions are called _______.
(Multiple Choice)
4.8/5  (40)
(40)
Does propane (shown)or octane (C8H18)exhibit stronger dispersion forces? 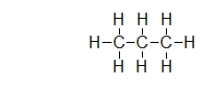
(Multiple Choice)
4.8/5  (43)
(43)
How many electron groups do the carbon atoms in ethylene (C2H4)have?
(Multiple Choice)
4.7/5  (36)
(36)
What is the molecular geometry of the atoms bonded to oxygen in methanol, shown below? 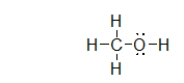
(Multiple Choice)
4.9/5  (41)
(41)
What is the angle between groups of electrons for an atom that has a linear electron geometry?
(Multiple Choice)
4.7/5  (39)
(39)
______ is the sharing of electrons between two atoms and is much stronger than intermolecular forces of attraction.
(Multiple Choice)
4.8/5  (30)
(30)
An atom, X, has a tetrahedral electron geometry but a trigonal pyramidal molecular geometry.How many atoms is atom X bonded to?
(Multiple Choice)
4.8/5  (47)
(47)
What is the angle between groups of electrons for an atom that has a tetrahedral electron geometry?
(Multiple Choice)
4.9/5  (36)
(36)
Antiestrogens are one type of molecule that can be used to treat breast cancer.Which of the following characteristics should be included in the design of a novel antiestrogen?
(Multiple Choice)
4.7/5  (37)
(37)
What is the molecular geometry of nitrogen trichloride (NCl3)?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 61 - 80 of 86
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)