Exam 38: Quantization
Exam 1: Concepts of Motion52 Questions
Exam 2: Kinematics in One Dimension59 Questions
Exam 3: Vectors and Coordinate Systems33 Questions
Exam 4: Kinematics in Two Dimensions50 Questions
Exam 5: Force and Motion30 Questions
Exam 6: Dynamics I: Motion Along a Line46 Questions
Exam 7: Newtons Third Law43 Questions
Exam 8: Dynamics Ii: Motion in a Plane20 Questions
Exam 9: Work and Kinetic Energy66 Questions
Exam 10: Interactions and Potential Energy55 Questions
Exam 11: Impulse and Momentum43 Questions
Exam 12: Rotation of a Rigid Body116 Questions
Exam 13: Newtons Theory of Gravity50 Questions
Exam 14: Fluids and Elasticity72 Questions
Exam 15: Oscillations49 Questions
Exam 16: Traveling Waves51 Questions
Exam 17: Superposition51 Questions
Exam 18: A Macroscopic Description of Matter46 Questions
Exam 19: Work, Heat, and the First Law of Thermodynamics96 Questions
Exam 20: The Micromacro Connection41 Questions
Exam 21: Heat Engines and Refrigerators44 Questions
Exam 22: Electric Charges and Forces26 Questions
Exam 23: The Electric Field32 Questions
Exam 24: Gausss Law41 Questions
Exam 25: The Electric Potential40 Questions
Exam 26: Potential and Field57 Questions
Exam 27: Current and Resistance32 Questions
Exam 28: Fundamentals of Circuits68 Questions
Exam 29: The Magnetic Field83 Questions
Exam 30: Electromagnetic Induction66 Questions
Exam 31: Electromagnetic Fields and Waves52 Questions
Exam 32: Ac Circuits44 Questions
Exam 33: Wave Optics51 Questions
Exam 34: Ray Optics60 Questions
Exam 35: Optical Instruments52 Questions
Exam 36: Relativity49 Questions
Exam 37: The Foundations of Modern Physics7 Questions
Exam 38: Quantization45 Questions
Exam 39: Wave Functions and Uncertainty18 Questions
Exam 40: One-Dimensional Quantum Mechanics32 Questions
Exam 41: Atomic Physics41 Questions
Exam 42: Nuclear Physics65 Questions
Select questions type
The Bohr radius of the hydrogen atom is 0.529 × 10-10 m. What is the radius of the n = 2 state?
(Multiple Choice)
4.8/5  (43)
(43)
The energy of the ground state in the Bohr model of the hydrogen atom is -13.6 eV. In a transition from the n = 2 state to the n = 4 state, a photon of energy
(Multiple Choice)
4.8/5  (39)
(39)
Upon being struck by 240-nm photons, a metal ejects electrons with a maximum kinetic energy of 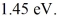 What is the work function of this metal? (h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
What is the work function of this metal? (h = 6.626 × 10-34 J ∙ s, c = 3.00 × 108 m/s, 1 eV = 1.60 × 10-19 J)
(Multiple Choice)
4.8/5  (35)
(35)
Calculate the kinetic energy (in eV) of a nonrelativistic neutron that has a de Broglie wavelength of 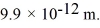 (h = 6.626 × 10-34 J ∙ s, mneutron = 1.675 × 10-27 kg,
1 eV = 1.60 × 10-19 J)
(h = 6.626 × 10-34 J ∙ s, mneutron = 1.675 × 10-27 kg,
1 eV = 1.60 × 10-19 J)
(Short Answer)
4.9/5  (32)
(32)
Light excites atomic hydrogen from its lowest level to the n = 4 level. What is the energy of the light? The energy of the lowest level is -13.6 eV.
(Multiple Choice)
4.8/5  (44)
(44)
Showing 41 - 45 of 45
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)