Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
Two containers of equal volume each hold samples of the same ideal gas. Container A has twice as many molecules as container B. If the gas pressure is the same in the two containers, the correct statement regarding the absolute temperatures TA and TB in containers A and B, respectively, is
(Multiple Choice)
4.9/5  (40)
(40)
A car starts out when the air temperature is 288 K and the absolute (total)air pressure in the tires is 500 kPa. After driving a while, the temperature of the air in the tires increases to 298 K. What is the pressure in the tires at that point, assuming their volume does not change?
(Multiple Choice)
4.7/5  (31)
(31)
A 360-g metal container, insulated on the outside, holds 180.0 g of water in thermal equilibrium at 22.0°C. A 24.0-g ice cube, at the melting point, is dropped into the water, and when thermal equilibrium is reached the temperature is 15.0°C. Assume there is no heat exchange with the surroundings. For water, the specific heat capacity is 4190 J/kg ∙ K and the heat of fusion is 3.34 × 105 J/kg. What is the specific heat capacity of the metal of the container?
(Multiple Choice)
4.9/5  (44)
(44)
A sample of ideal monatomic gas is cooled by 50.0 C° at constant volume by removing 831 J of energy from it. How many moles of gas are in the sample? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (42)
(42)
An aluminum rod 17.400 cm long at 20°C is heated to 100°C. What is its new length? Aluminum has a linear expansion coefficient of 25 × 10-6 K-1.
(Multiple Choice)
4.8/5  (35)
(35)
A solid cylindrical bar conducts heat at a rate of 25 W from a hot to a cold reservoir under steady state conditions. If both the length and the diameter of this bar are doubled, the rate at which it will conduct heat between these reservoirs will be
(Multiple Choice)
4.8/5  (34)
(34)
An ideal gas has a pressure of 2.5 atm, a volume of 1.0 L at a temperature of 30°C. How many molecules are there in this gas? (R = 8.31 J/mol ∙ K,1.00 atm = 101 kPa, NA = 6.022 × 1023)
(Multiple Choice)
4.7/5  (33)
(33)
A 24.0-L tank contains ideal helium gas at 27°C and a pressure of 22.0 atm. How many moles of gas are in the tank? (R = 8.31 J/mol ∙ K, 1 atm = 101 kPa)
(Multiple Choice)
4.9/5  (32)
(32)
Two processes are shown on the pV diagram in the figure. One of them is an adiabat and the other one is an isotherm. Which process is the isotherm? 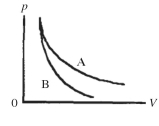
(Multiple Choice)
5.0/5  (32)
(32)
Originally 2.00 mol of gas are at STP. If the temperature changes to 47.0°C and the pressure decreases to half of what it was, how many liters do the two moles now occupy? (1 atm = 101 kPa, R = 8.31 J/mol ? K)
(Short Answer)
4.7/5  (42)
(42)
A gas expands from an initial volume of 30.0 L to a final volume of 65.0 L at a constant pressure of 110 kPa. How much work is done by the gas during this expansion?
(Multiple Choice)
4.9/5  (37)
(37)
A hole in a brass plate has a diameter of 1.200 cm at 20°C. What is the diameter of the hole when the plate is heated to 220°C? The coefficient of linear thermal expansion for brass is 19 × 10-6 K-1.
(Multiple Choice)
4.9/5  (34)
(34)
A jar holds 2.0 L of ideal nitrogen gas, N2, at STP. The atomic mass of nitrogen is 14.0 g/mol, the ideal gas constant is R = 8.31 J/mol ? K, Avogadro's number is NA = 6.022 × 1023 molecules/mol, and 1.00 atm = 101 kPa.
(a)How many moles of nitrogen are in the jar?
(b)How many nitrogen molecules are in the jar?
(c)What is the mass of the nitrogen in the jar?
(Short Answer)
4.9/5  (42)
(42)
If the absolute temperature of an object is tripled, the thermal power radiated by this object (assuming that its emissivity and size are not affected by the temperature change)will
(Multiple Choice)
4.9/5  (43)
(43)
If you wanted to know how much the temperature of a particular piece of material would rise when a known amount of heat was added to it, which of the following quantities would be most helpful to know?
(Multiple Choice)
4.9/5  (38)
(38)
Oxygen molecules are 16 times more massive than hydrogen molecules. At a given temperature, the average molecular kinetic energy of oxygen molecules, compared to that of hydrogen molecules,
(Multiple Choice)
4.7/5  (37)
(37)
A 40.0-g block of ice at -15.00°C is dropped into a calorimeter (of negligible heat capacity)containing water at 15.00°C. When equilibrium is reached, the final temperature is 8.00°C. How much water did the calorimeter contain initially? The specific heat of ice is 2090 J/kg ∙ K, that of water is 4186 J/kg ∙ K, and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Multiple Choice)
4.9/5  (39)
(39)
A sphere of surface area 1.25 m2 and emissivity 1.0 is at a temperature of 100°C. At what rate does it radiate heat into empty space? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (41)
(41)
A 0.600-kg piece of metal X is heated to 100°C and placed in an aluminum can of mass 0.200-kg which contains 0.500 kg of water initially at 17.3°C. The final equilibrium temperature of the mixture is 20.2°C, what is the specific heat of metal X? The specific heats of water and aluminum are 4186 J/kg ∙ K (water)and 910 J/kg ∙ K (aluminum).
(Multiple Choice)
4.8/5  (34)
(34)
The coefficient of linear expansion of copper is 17 × 10-6 K-1. A sheet of copper has a round hole with a radius of 3.0 m cut out of it. If the sheet is heated and undergoes a change in temperature of 80 K, what is the change in the radius of the hole?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 61 - 80 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)