Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
How much power does a sphere with a radius of 10 cm radiate into empty space if is has an emissivity of 1.0 and is kept at a temperature of 400 K? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (36)
(36)
A glass flask has a volume of 500 mL at a temperature of 20° C. The flask contains 492 mL of mercury at an equilibrium temperature of 20°C. The temperature is raised until the mercury reaches the 500 mL reference mark. At what temperature does this occur? The coefficients of volume expansion of mercury and glass are 18 ×10-5 K-1 (mercury)and 2.0 ×10-5 K-1 (glass).
(Multiple Choice)
4.9/5  (28)
(28)
The absolute temperature of a gas is T. In order to double the rms speed of its molecules, what should be the new absolute temperature?
(Multiple Choice)
4.9/5  (47)
(47)
The coefficient of linear expansion of aluminum is 24.0 × 10-6 K-1, and the density of aluminum at 0°C is 2.70 × 103 kg/m3. What is the density of aluminum at 300°C?
(Multiple Choice)
4.8/5  (30)
(30)
A sealed container holds 0.020 moles of ideal nitrogen (N2)gas, at a pressure of 1.5 atm and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. How many molecules of nitrogen are in the container? (R = 8.31 J/mol ∙ K, 1 atm = 101 kPa)
(Multiple Choice)
4.8/5  (30)
(30)
The coefficient of linear expansion of copper is 17 × 10-6 K-1 and that of steel is 12 × 10-6 K-1. At 12°C a steel rod has a diameter of 2.540 cm and a copper pipe has a diameter of 2.536 cm. Which one of the following quantities is closest to the temperature to which the copper pipe must be heated in order for the unheated steel rod to fit snugly in the copper pipe?
(Multiple Choice)
4.8/5  (43)
(43)
A gas is taken through the cycle shown in the pV diagram in the figure. During one cycle, how much work is done by the gas? 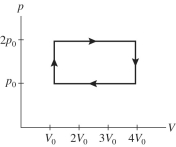
(Multiple Choice)
4.7/5  (39)
(39)
Two experimental runs are performed to determine the calorimetric properties of an alcohol which has a melting point of -10.0° C. In the first run, a 200-g cube of frozen alcohol, at the melting point, is added to 300 g of water at 20.0°C in a styrofoam container. When thermal equilibrium is reached, the alcohol-water solution is at a temperature of 5.0°C. In the second run, an identical cube of alcohol is added to 500 g of water at 20.0°C and the temperature at thermal equilibrium is 10.0°C. The specific heat capacity of water is 4190 J/kg ∙ K. Assume no heat is exchanged with the styrofoam container and the surroundings. What is the heat of fusion of the alcohol?
(Multiple Choice)
4.8/5  (28)
(28)
When 50 g of a certain material at 100°C is mixed with 100 g of water at 0°C, the final temperature is 40°C. What is the specific heat of the material? The specific heat of water is 1.00 kcal/kg ∙ C°.
(Multiple Choice)
4.8/5  (30)
(30)
A certain automobile tire has a volume of 0.0185 m3. If the absolute (or total)pressure in the tire is 500 kPa and the temperature is 298 K, how many molecules are there inside the tire? (R = 8.31 J/mol ∙ K, NA = 6.022 x 1023 molecules/mol)
(Multiple Choice)
4.8/5  (38)
(38)
Dust particles in a grain elevator frequently have masses of the order of 1.0 × 10-9 kg. If, to a first approximation, we model the dust particles as an ideal gas, what would be the rms speed of such a particle in air at 27°C? The Boltzmann constant is 1.38 × 10-23 J/K .
(Multiple Choice)
4.9/5  (37)
(37)
A 6.5-g iron meteor hits the earth at a speed of 295 m/s. If its kinetic energy is entirely converted to heat in the meteor, by how much will its temperature rise? The specific heat of iron is 113 cal/kg ∙ C°, and 1 cal = 4.186 J.
(Multiple Choice)
4.9/5  (35)
(35)
A person is walking outdoors on a cold day when the temperature is -20°C. He is breathing at the rate of 16 breaths per minute, and each time he breathes in he inhales 0.0050 m3 of air. At what rate does he lose heat from breathing if the air in his lungs is heated to body temperature (37°C)before it is exhaled? The specific heat of air is 1020 J/kg ∙ K and the density of air is 1.29 kg/m3.
(Multiple Choice)
4.9/5  (44)
(44)
A flask contains a mixture of argon and neon gases at a stabilized temperature. The root-mean-square speed of the argon gas is determined to be 1.21 km/s. What is the root-mean-square speed of the neon gas? The atomic mass of argon is 39.95 g/mol, and that of neon is 20.18 g/mol.
(Short Answer)
4.8/5  (37)
(37)
The absolute temperature of an ideal gas is directly proportional to which of the following quantities?
(Multiple Choice)
4.9/5  (34)
(34)
On a cold day, a piece of metal feels much colder to the touch than a piece of wood. This is due to the difference in which one of the following physical properties of these materials?
(Multiple Choice)
4.9/5  (42)
(42)
How much heat is required to increase the temperature of 1.70 moles of an ideal monatomic gas by 23.0 K at constant pressure? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (40)
(40)
At what temperature would the root mean square speed of oxygen molecules, O2, be if oxygen behaves like an ideal gas? The mass of one O2 molecule is 5.312 × 10-26 kg, and the Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (37)
(37)
What is the net power radiated by a little animal with a surface area of 0.075 m2 if his emissivity is 0.75, his skin temperature is 315 K, and he is in a room with a temperature of 290 K? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (37)
(37)
Showing 81 - 100 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)