Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
The coefficient of linear expansion of aluminum is 24 × 10-6 K-1 and the coefficient of volume expansion of olive oil is 0.68 × 10-3 K-1. A novice cook, in preparation of some pesto, fills a 1.00-L aluminum pot to the brim and heats the oil and the pot from an initial temperature of 15°C to 190°C. To his consternation some olive oil spills over the top. How much?
(Multiple Choice)
4.9/5  (37)
(37)
Oxygen molecules are 16 times more massive than hydrogen molecules. At a given temperature, how do their average molecular speeds compare? The oxygen molecules are moving
(Multiple Choice)
4.8/5  (36)
(36)
A mole of diatomic oxygen molecules and a mole of diatomic nitrogen molecules are at STP. Which statements are true about these molecules? (There could be more than one correct choice.)
(Multiple Choice)
4.8/5  (31)
(31)
An ideal gas occupies 6.00 × 102 cm3 at 20°C. At what temperature will it occupy 1.20 × 103 cm3 if the pressure is held constant?
(Multiple Choice)
4.7/5  (38)
(38)
A gas-filled vertical cylinder, closed at the bottom end, is fitted at the top with a piston that can move freely. The mass of the piston is 10.0 kg, and the initial height of the piston above the bottom of the cylinder is 25 cm. A mass of 8.0 kg is placed on the piston. What is the resulting height of the piston, assuming that the temperature of the ideal gas is kept constant?
(Multiple Choice)
4.8/5  (37)
(37)
A large vat contains 1.000 L of water at 20°C. What volume will this water occupy when it is heated up to 80°C? Water has a volume expansion coefficient of 210 × 10-6 K-1.
(Multiple Choice)
4.7/5  (31)
(31)
For the mercury in a thermometer to expand from 4.00 cm3 to 4.10 cm3, what change in temperature is necessary? The mercury has a volume expansion coefficient of 1.80 × 10-4 K-1.
(Multiple Choice)
4.8/5  (35)
(35)
The figure shows a pV diagram for 0.98 mol of ideal gas that undergoes the process 1 → 2. The gas then undergoes an isochoric heating from point 2 until the pressure is restored to the value it had at point 1. What is the final temperature of the gas? (R = 8.31 J/mol ∙ K). 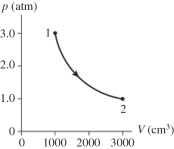
(Multiple Choice)
4.8/5  (35)
(35)
A substance has a melting point of 20°C and a heat of fusion of The boiling point is and the heat of vaporization is at a pressure of one atmosphere. The specific heats for the solid, liquid, and gaseous phases are 600 J/kg ? K (solid), 1000 J/kg ? K (liquid), and 400 J/kg ? K (gaseous). How much heat is given up by of this substance when it is cooled from 170°C to 86°C at a pressure of one atmosphere?
(Multiple Choice)
4.9/5  (45)
(45)
A refrigerator has an interior volume of 0.500 m3. The temperature inside the refrigerator in 282 K, and the pressure is 101 kPa. If the molecular weight of air is 29 g/mol, what is the mass of air inside the refrigerator? (R = 8.31 J/mol × K)
(Multiple Choice)
4.7/5  (35)
(35)
A camper is about to drink his morning coffee. He pours 400 grams of coffee, initially at 75°C into a 250-g aluminum cup, initially at 16°C. What is the equilibrium temperature of the coffee-cup system, assuming no heat is lost to the surroundings? The specific heat of aluminum is 900 J/kg ∙ K, and the specific heat of coffee is essentially the same as that of water, which is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (39)
(39)
On his honeymoon, James Joule attempted to explore the relationships between various forms of energy by measuring the rise of temperature of water which had fallen down a waterfall on Mount Blanc. What maximum temperature rise would one expect for a waterfall with a vertical drop of 20 m? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (41)
(41)
The coefficient of linear expansion of copper is 17 × 10-6 K-1. A block of copper 30 cm wide, 45 cm long, and 10 cm thick is heated from 0°C to 100°C What is the change in the volume of the block?
(Multiple Choice)
4.8/5  (30)
(30)
A lab assistant pours 330 g of water at 45°C into an 855-g aluminum container that is at an initial temperature of 10°C. The specific heat of aluminum is and that of water is 4186 J/kg ? K. What is the final temperature of the system, assuming no heat is exchanged with the surroundings?
(Multiple Choice)
4.8/5  (39)
(39)
An aluminum rod is 10.0 cm long and a steel rod is 80.0 cm long when both rods are at a temperature of 15°C. Both rods have the same diameter. The rods are now joined end-to-end to form a rod 90.0 cm long. If the temperature is now raised from 15°C to 90°C, what is the increase in the length of the joined rod? The coefficient of linear expansion of aluminum is 2.4 × 10-5 K-1 and that of steel is 1.2 × 10-5 K-1.
(Multiple Choice)
4.8/5  (35)
(35)
A vertical cylinder, closed at the bottom end, contains 0.0100 mol of ideal gas. It is fitted at the top with a piston that can move freely. The mass of the piston is 14.0 kg and the initial height of the piston above the bottom of the cylinder is 25 cm. What is the temperature of the gas? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (42)
(42)
A gas expands from an initial volume of 0.040 m3 to a final volume of 0.085 m3 while its pressure increases linearly with the volume (so that the process follows a straight-line path in a pV diagram)from 110 kPa to 225 kPa. How much work is done by the gas during this expansion?
(Multiple Choice)
4.8/5  (31)
(31)
If you add 1.33 MJ of heat to 500 g of water at 50°C in a sealed container, what is the final temperature of the steam? The latent heat of vaporization of water is 22.6 × 105 J/kg, the specific heat of steam is 2010 J/kg ∙ K, and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.7/5  (40)
(40)
Showing 181 - 200 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)