Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
Two metal rods, one silver and the other gold, are attached to each other end-to-end. The free end of the silver rod is immersed in a steam chamber at 100°C, and the free end of the gold rod in an ice water bath at 0°C. The rods are both 5.0 cm long and have a square cross-section that is 2.0 cm on a side. No heat is exchanged between the rods and their surroundings, except at the ends.
- How much total heat flows through the two rods each minute? The thermal conductivity of silver is 417 W/m ? K, and that of gold is 291 W/m ? K.
(Multiple Choice)
4.8/5  (28)
(28)
An ideal gas undergoes the process a→b→c→a shown in the pV diagram. In this figure, Pa = Pc = 3.60 × 105 Pa, Vb = Vc = 68.00 L, Va = 35 L, and Pb = 5.60 × 105 Pa. How much work is done by the system in this process? 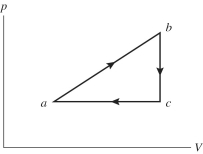
(Multiple Choice)
4.9/5  (34)
(34)
A sealed container holds 0.020 moles of ideal nitrogen (N2)gas, at a pressure of 1.5 atm and a temperature of 290 K. The atomic mass of nitrogen is 14.0 g/mol. What is the average translational kinetic energy of a nitrogen molecule? The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (34)
(34)
A glass beaker of unknown mass contains of water. The system absorbs of heat and the temperature rises as a result. What is the mass of the beaker? The specific heat of glass is 0.18 cal/g ? °C, and that of water is 1.0 cal/g ? C°.
(Multiple Choice)
4.8/5  (49)
(49)
A 20.0-L pressure vessel holds 2.00 mol of oxygen at 30°C. What is the pressure inside the vessel? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (34)
(34)
If 50 g of lead (of specific heat 0.11 kcal/kg ∙ C°)at 100°C is put into 75 g of water (of specific heat 1.0 kcal/kg ∙ C°)at 0°C. What is the final temperature of the mixture?
(Multiple Choice)
4.8/5  (35)
(35)
A sample of an ideal gas is heated and its Kelvin temperature doubles. If the root-mean-square speed of its molecules was originally v, what is the new root-mean-square speed?
(Multiple Choice)
4.7/5  (34)
(34)
If we add 700 J of heat to 12 moles of an ideal monatomic gas at constant volume, what will be the change in temperature of the gas? (R = 8.31 J/mol ∙ K)
(Multiple Choice)
4.8/5  (35)
(35)
Consider two equal-volume flasks of gas at the same temperature and pressure. One gas, oxygen, has a molecular mass of 32. The other gas, nitrogen, has a molecular mass of 28. What is the ratio of the number of oxygen molecules to the number of nitrogen molecules in these flasks?
(Multiple Choice)
4.8/5  (35)
(35)
A heat-conducting rod that is wrapped in insulation is constructed with a 0.15-m length of alloy A and a 0.40-m length of alloy B, joined end-to-end. Both pieces have cross-sectional areas of 0.0020 m2. The thermal conductivity of alloy B is known to be 1.8 times as great as that for alloy A. The end of the rod in alloy A is maintained at a temperature of 10°C, and the other end of the rod is maintained at an unknown temperature. When steady state flow has been established, the temperature at the junction of the alloys is measured to be 40° C, and the rate of heat flow in the rod is measured at 56 W.
- What is the thermal conductivity of alloy A?
(Multiple Choice)
4.9/5  (33)
(33)
A 4.2-L flask of ideal neon gas (which is monatomic)is at a pressure of 3.3 atm and a temperature of 450 K. The atomic mass of neon is 20.2 g/mol. How many neon atoms are in the flask? (R = 8.31 J/mol ∙ K, 1 atm = 101 kPa, NA = 6.022 × 1023 molecules/mol)
(Multiple Choice)
4.8/5  (33)
(33)
The water flowing over Niagara Falls drops a distance of 50 m. If all the gravitational potential energy is converted to thermal energy, by what temperature does the water rise? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.7/5  (39)
(39)
A balloon originally has a volume of 1.0 m3 when the gas in it is at 20°C and under a pressure of 1.0 atm. As it rises in the earth's atmosphere, its volume expands. What will be its new volume if its final temperature and pressure are -40°C and 0.10 atm?
(Multiple Choice)
4.9/5  (38)
(38)
The figure shows a pV diagram for 0.0077 mol of ideal gas that undergoes the process 1 ? 2 ? 3. What is the volume V3? (R = 8.31 J/mol ? K) 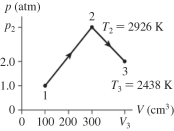 the volume V3?
the volume V3?
(Multiple Choice)
4.9/5  (33)
(33)
At what temperature would the root-mean-square speed of hydrogen, H2, molecules equal 11.2 km/s (the earth's escape speed)? The mass of a hydrogen atom is 1.67 × 10-27 kg, and the Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (39)
(39)
If a certain sample of an ideal gas has a temperature of 104°C and exerts a pressure of 2.3 × 104 Pa on the walls of its container, how many gas molecules are present in each cubic centimeter of volume? The ideal gas constant is R = 8.31 J/mol × K and Avogadro's number is NA = 6.022 × 1023 molecules/mol.
(Short Answer)
4.8/5  (36)
(36)
The volume coefficient of thermal expansion for gasoline is 950 × 10-6 K-1. By how many cubic centimeters does the volume of 1.00 L of gasoline change when the temperature rises from 30°C to 50°C?
(Multiple Choice)
4.8/5  (33)
(33)
A heat conducting rod, 1.60 m long and wrapped in insulation, is made of an aluminum section that is 0.90 m long and a copper section that is long. Both sections have a cross-sectional area of The aluminum end and the copper end are maintained at temperatures of and respectively. The thermal conductivities of aluminum and copper are 205 W/m ? K (aluminum)and 385 W/m ? K (copper). At what rate is heat conducted in the rod under steady state conditions?
(Multiple Choice)
4.7/5  (41)
(41)
A mercury thermometer has a glass bulb of interior volume 0.100 cm3 at 10°C. The glass capillary tube above the bulb has an inner cross-sectional area of 0.012 mm2. The coefficient of volume expansion of mercury is 1.8 × 10-4 K-1. If the expansion of the glass is negligible, how much will the mercury rise in the capillary tube when the temperature rises from 5°C to 35°C if the bulb was full at 5°C?
(Multiple Choice)
4.8/5  (35)
(35)
If, with steady state heat flow established, you double the thickness of a wall built from solid uniform material, the rate of heat loss for a given temperature difference across the thickness will
(Multiple Choice)
4.8/5  (35)
(35)
Showing 101 - 120 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)