Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A 200-L electric water heater uses 2.0 kW. Assuming no heat loss, how many hours would it take to heat the water in this tank from 23°C to 75°C? The specific heat of water is 4186 J/kg ∙ K and its density is 1000 kg/m3.
(Multiple Choice)
4.9/5  (29)
(29)
Object 1 has three times the specific heat capacity and four times the mass of Object 2. The two objects are given the same amount of heat. If the temperature of Object 1 changes by an amount ?T, the change in temperature of Object 2 will be
(Multiple Choice)
4.9/5  (36)
(36)
Suppose that a rigid aluminum wire were to be strung out in a loop that just fits snugly around the equator (assuming a perfectly spherical Earth with a radius of 6.37 × 106 m). If the temperature of the wire is increased by 0.50°C, and the increase in length is distributed equally over the entire length, how far off the ground will the wire loop be if it remained centered on the earth? The coefficient of linear expansion of aluminum is 24 × 10-6 K-1.
(Multiple Choice)
4.9/5  (42)
(42)
An architect is interested in estimating the rate of heat loss, ΔQ/Δt, through a sheet of insulating material as a function of the thickness of the sheet. Assuming fixed temperatures on the two faces of the sheet and steady state heat flow, which one of the graphs shown in the figure best represents the rate of heat transfer as a function of the thickness of the insulating sheet? 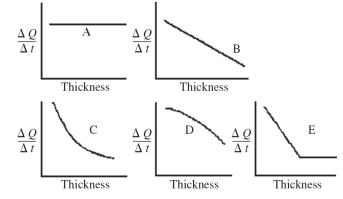
(Multiple Choice)
5.0/5  (41)
(41)
What is the total translational kinetic energy of the gas in a classroom filled with nitrogen at at The dimensions of the classroom are The Boltzmann constant is 1.3806503 × 10-23 J/K, R = 8.31 J/mol ? K, and NA = 6.022 × 1023 molecules/mol.
(Short Answer)
4.9/5  (39)
(39)
A metal has a latent heat of fusion of 2.32 × 104 J/kg, a specific heat of 128 J/kg ∙ K, and a melting point of 228°C. A 30-g pellet of this metal at 16°C hits a solid wall and comes to a complete stop. What would the speed of the pellet have to be in order for it to melt completely when it hits the wall, assuming that all of its kinetic energy is transformed into heat within the pellet?
(Multiple Choice)
4.8/5  (39)
(39)
A beaker of negligible heat capacity contains 456 g of ice at -25.0°C. A lab technician begins to supply heat to the container at the rate of 1000 J/min. How long after starting will the ice begin to melt, assuming all of the ice has the same temperature? The specific heat of ice is 2090 J/kg ? K and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Short Answer)
4.8/5  (36)
(36)
A certain ideal gas has a molar specific heat at constant pressure of 7R/2. What is its molar specific heat at constant volume?
(Multiple Choice)
4.8/5  (40)
(40)
A person makes iced tea by adding ice to 1.8 kg of hot tea, initially at 80°C. How many kilograms of ice, initially at 0°C, are required to bring the mixture to 10°C? The specific heat of water (and tea)is 4186 J/kg ∙ K, and the latent heat of fusion of ice is 3.34 × 105 J/kg.
(Multiple Choice)
4.8/5  (35)
(35)
What is the net power that a person with surface area of 1.20 m2 radiates if his emissivity is 0.895, his skin temperature is 27°C, and he is in a room that is at a temperature of 17°C? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (44)
(44)
If the temperature of a gas is increased from 20°C to 100°C, by what factor does the rms speed of an ideal molecule change?
(Multiple Choice)
4.9/5  (43)
(43)
Consider a flat steel plate with a hole through its center as shown in the figure. When the temperature of the plate is increased, the hole will 
(Multiple Choice)
4.9/5  (44)
(44)
A certain ideal gas has a molar specific heat at constant pressure of 33.2 J/mol ∙ K. Its molar specific heat at constant volume is closest to which of the following values? (R = 8.31J/mol ∙ K)
(Multiple Choice)
4.8/5  (37)
(37)
A person tries to heat up her bath water by adding 5.0 L of water at 80°C to 60 L of water at 30°C. What is the final temperature of the bath water?
(Multiple Choice)
4.9/5  (33)
(33)
A solid concrete wall has dimensions 4.0 m × 2.4 m and is 30 cm thick. The thermal conductivity of the concrete is 1.3 W/m ∙ K, and it separates a basement from the ground outside. The inner surface of the wall is at 18°C, and the outside surface is at 6°C. How much heat flows through the wall every hour?
(Multiple Choice)
4.8/5  (35)
(35)
An ideal gas is held in a container of volume V at pressure p. The rms speed of a gas molecule under these conditions is v. If now the volume and pressure are changed to 2V and 2p, the rms speed of a molecule will be
(Multiple Choice)
4.8/5  (37)
(37)
Two metal spheres are made of the same material and have the same diameter, but one is solid and the other is hollow. If their temperature is increased by the same amount,
(Multiple Choice)
4.7/5  (32)
(32)
The figure shows a graph of the temperature of a pure substance as a function of time as heat is added to it at a constant rate in a closed container. If LF is the latent heat of fusion of this substance and LV is its latent heat of vaporization, what is the value of the ratio LV/LF? 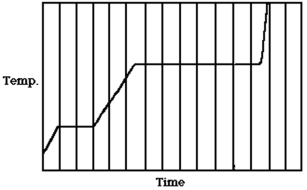
(Multiple Choice)
4.8/5  (38)
(38)
An object having a fixed emissivity of 0.725 radiates heat at a rate of 10 W when it is at an absolute temperature T. If its temperature is doubled to 2T, at what rate will it now radiate?
(Multiple Choice)
4.8/5  (41)
(41)
A carpenter is driving a 15.0-g steel nail into a board. His 1.00-kg hammer is moving at 8.50 m/s when it strikes the nail. Half of the kinetic energy of the hammer is transformed into heat in the nail and does not flow out of the nail. What is the increase in temperature of the nail after the three blows that the carpenter needs to drive the nail in completely? The specific heat of steel is 448 J/kg ∙ K.
(Multiple Choice)
4.9/5  (41)
(41)
Showing 121 - 140 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)