Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
(a)At what Celsius temperature is the average kinetic energy of a helium gas atom equal to 6.21 × 10-21 J? The Boltzmann constant is 1.38 × 10-23 J/K .
(b)What would be the temperature for radon gas?
(Short Answer)
4.9/5  (42)
(42)
A 771.0-kg copper bar is put into a smelter for melting. The initial temperature of the copper is 300.0 K. How much heat must the smelter produce to completely melt the copper bar? The specific heat for copper is 386 J/kg∙K, the heat of fusion for copper is 205,000 J/kg, and its melting point is 1357 K.
(Multiple Choice)
4.8/5  (36)
(36)
The melting point of aluminum is 660°C, its latent heat of fusion is 4.00 × 105 J/kg, and its specific heat is 900J/kg ∙ K. How much heat must be added to 500 g of aluminum originally at 27°C to completely melt it?
(Multiple Choice)
4.9/5  (36)
(36)
The figure shows a pV diagram for 2.6 g of ideal helium gas that undergoes the process 1 → 2 → 3. Find the value of volume V3. The atomic mass of helium is 4.0 g/mol, and R = 8.31 J/mol ∙ K. 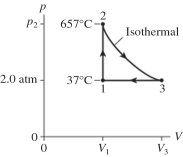
(Multiple Choice)
4.7/5  (36)
(36)
A glass tea kettle containing 500 g of water is on the stove. The portion of the tea kettle that is in contact with the heating element has an area of 0.090 m2 and is 1.5 mm thick. At a certain moment, the temperature of the water is 75°C, and it is rising at the rate of 3 C° per minute. What is the temperature of the outside surface of the bottom of the tea kettle? Neglect the heat capacity of the kettle, and assume that the inner surface of the kettle is at the same temperature as the water inside. The thermal conductivity of glass is 0.840 W/m ∙ K and the specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (30)
(30)
A 400-g block of iron at 400°C is dropped into a calorimeter (of negligible heat capacity)containing 60 g of water at 30°C. How much steam is produced? The latent heat of vaporization of water is 22.6 × 105 J/kg and its specific heat capacity is 4186 J/kg ∙ K. The average specific heat of iron over this temperature range is 560 J/kg ∙ K.
(Multiple Choice)
4.8/5  (41)
(41)
A runner generates 1260 W of thermal energy. If this heat has to be removed only by evaporation, how much water does this runner lose in 15 minutes of running? The latent heat of vaporization of water is 22.6 × 105 J/kg.
(Multiple Choice)
4.8/5  (36)
(36)
An ideal gas is compressed isothermally to one-third of its initial volume. The resulting pressure will be
(Multiple Choice)
4.7/5  (41)
(41)
How many molecules are in (a)1.0 cm3 of air at STP and (b)1.0 cm3 of helium at STP? (R = 8.31 J/mol . K, NA = 6.022 × 1023 molecules/mol)
(Essay)
4.8/5  (38)
(38)
What is the average translational kinetic energy of an ideal gas at The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.8/5  (48)
(48)
A 90-g aluminum calorimeter contains 390 g of water at an equilibrium temperature of A piece of metal, initially at is added to the calorimeter. The final temperature at equilibrium is 32° C. Assume there is no external heat exchange. The specific heat capacities of aluminum and water are 910 J/kg ? K (aluminum)and 4190 J/kg ? K (water). What is the specific heat capacity of the 160-g piece of metal?
(Multiple Choice)
4.9/5  (37)
(37)
A 400-g stainless steel tea kettle containing 500 g of water is on the stove. The portion of the tea kettle that is in contact with the heating element has an area of 0.090 m2 and is 2.0 mm thick. At a certain moment, the temperature of the water is 75°C, and it is rising at the rate of 3.0 C° per minute. What is the difference in temperature between the inside and the outside of the bottom of the tea kettle? Assume that the inner surface of the kettle is at the same temperature as the water inside. The thermal conductivity of stainless steel is 16.3 W/m ∙ K, the specific heat of the steel is 448 J/kg ∙ K, and the specific heat of water is 4186 J/kg . K.
(Multiple Choice)
4.9/5  (35)
(35)
A beaker of negligible heat capacity contains 456 g of ice at -25.0°C. A lab technician begins to supply heat to the container at the rate of 1000 J/min. How long after starting will it take before the temperature starts to rise above 0°C? The specific heat of ice is 2090 J/kg ? K and the latent heat of fusion of water is 33.5 × 104 J/kg.
(Short Answer)
4.7/5  (38)
(38)
A lab student drops a 400.0-g piece of metal at 120.0°C into a cup containing 450.0 g of water at 15.0°C. After waiting for a few minutes, the student measures that the final temperature of the system is 40.0°C. What is the specific heat of the metal, assuming that no significant heat is exchanged with the surroundings or the cup? The specific heat of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.8/5  (39)
(39)
The figure shows a pV diagram for 0.0061 mol of ideal gas that undergoes the process 1 → 2 → 3. What is the pressure p2? (R = 8.31 J/mol ∙ K) 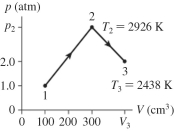
(Multiple Choice)
4.8/5  (35)
(35)
A concrete wall of a cold storage room measures 3.0 m high, 5.0 m wide, and 20 cm thick. The inside wall is to be covered by a layer of wood in order to reduce the rate of heat flow through the wall by 90 percent. The inner surface of the wooden wall is maintained at -10°C and the outer surface of the concrete wall is at 20°C. The thermal conductivities of concrete and wood are 0.80 W/m ? K (concrete)and 0.040 W/m ? K (wood).
-What should be the thickness of the layer of wood?
(Multiple Choice)
4.9/5  (38)
(38)
The melting point of aluminum is 660°C, its latent heat of fusion is 4.00 × 105 J/kg, and its specific heat is 900 J/kg ∙ K. If 300 kJ of heat are added to 442 g of aluminum at 100°C, what is the final state of the system? That is, how much is liquid, how much is solid, and what is its temperature?
(Essay)
5.0/5  (43)
(43)
The coefficient of linear expansion of copper is 17 × 10-6 K-1 and that of steel is 12 × 10-6 K-1. At 12°C a steel rod has a diameter of 2.540 cm and a copper pipe has a diameter of 2.536 cm. If they are heated together to a higher temperature, which one of the following quantities is closest to the common temperature at which the steel rod will fit snugly in the copper pipe?
(Multiple Choice)
4.8/5  (40)
(40)
The cylindrical filament in a light bulb has a diameter of 0.050 mm, an emissivity of 1.0, and a temperature of 3000°C. How long should the filament be in order to radiate 60 W of power? (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.8/5  (34)
(34)
Showing 21 - 40 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)