Exam 12: Thermal Properties of Matter
Exam 1: Representing Motion113 Questions
Exam 2: Motion in One Dimension172 Questions
Exam 3: Vectors and Motion in Two Dimensions180 Questions
Exam 4: Forces and Newtons Laws of Motion64 Questions
Exam 5: Applying Newtons Laws82 Questions
Exam 6: Gravity96 Questions
Exam 7: Rotational Motion95 Questions
Exam 8: Equilibrium Ad Elasticity72 Questions
Exam 9: Momentum103 Questions
Exam 10: Energy and Work219 Questions
Exam 11: Using Energy106 Questions
Exam 12: Thermal Properties of Matter220 Questions
Exam 13: Fluids112 Questions
Exam 14: Oscillations105 Questions
Exam 15: Traveling Waves and Sound94 Questions
Exam 16: Superposition and Standing Waves66 Questions
Exam 17: Wave Optics129 Questions
Exam 18: Ray Optics154 Questions
Exam 19: Optical Instruments137 Questions
Exam 20: Electric Fields and Forces86 Questions
Exam 21: Electric Potential140 Questions
Exam 22: Current and Resistance124 Questions
Exam 23: Circuits145 Questions
Exam 24: Magnetic Fields and Forces155 Questions
Exam 25: Em Induction and Em Waves184 Questions
Exam 26: Ac Electricity122 Questions
Exam 27: Relativity125 Questions
Exam 28: Quantum Physics85 Questions
Exam 29: Atoms and Molecules105 Questions
Exam 30: Nuclear Physics175 Questions
Select questions type
A 4.0-kg aluminum block is originally at 10°C. If 160 kJ of heat is added to the block, what is its final temperature? The specific heat capacity of aluminum is 910 J/kg ∙ K.
(Multiple Choice)
4.8/5  (30)
(30)
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper, with the aluminum and the copper in thermal contact. The specific heat capacity of aluminum is more than double that of copper.
- Which object experiences the greater temperature change during the time the system takes to reach thermal equilibrium?
(Multiple Choice)
4.9/5  (45)
(45)
A machine part consists of 0.10 kg of iron (of specific heat 470 J/kg ∙ K )and 0.16 kg of copper (of specific heat 390 J/kg ∙ K). How much heat must be added to the gear to raise its temperature from 18°C to 53°C?
(Multiple Choice)
4.9/5  (43)
(43)
On a cold day, you take in 4.2 L of air into your lungs at a temperature of 0°C. If you hold your breath until the temperature of the air in your lungs reaches 37°C, what is the volume of the air in your lungs at that point, assuming the pressure does not change?
(Multiple Choice)
4.9/5  (42)
(42)
A sealed cylinder fitted with a movable piston contains ideal gas at 27°C, pressure 0.500 × 105 Pa, and volume 1.25 m3. What will be the final temperature if the gas is compressed to 0.800 m3 and the pressure rises to 0.820 × 105 Pa?
(Multiple Choice)
4.9/5  (37)
(37)
A window glass that is 0.5 cm thick has dimensions of 3 m by 1.5 m. The thermal conductivity of this glass is 0.8 W/m ∙ K. If the outside surface of the glass is at -10°C and the inside surface is at 20°C, how much heat flows through the window in every hour?
(Multiple Choice)
4.8/5  (41)
(41)
A rigid container is filled with 4.0 mol of air with CV = 2.5R. How much does the internal (thermal)energy of the air change if its temperature rises from to (R = 8.31 J/mol ? K)
(Multiple Choice)
4.8/5  (33)
(33)
A thermally isolated system is made up of a hot piece of aluminum and a cold piece of copper, with the aluminum and the copper in thermal contact. The specific heat capacity of aluminum is more than double that of copper.
- Which object experiences the greater magnitude gain or loss of heat during the time the system takes to reach thermal equilibrium?
(Multiple Choice)
4.8/5  (45)
(45)
The figure shows a pV diagram for 8.3 g of ideal nitrogen gas N2 in a sealed container. The temperature of state 1 is 59°C, the atomic mass of the nitrogen atom is 14 g/mol, and R = 8.31 J/mol ∙ K. What are (a)pressure p1 and (b)temperature T2? 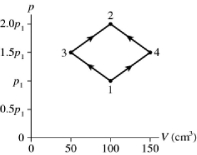
(Multiple Choice)
4.7/5  (29)
(29)
A quantity of an ideal gas is kept in a rigid container of constant volume. If the gas is originally at a temperature of 19°C, at what temperature will the pressure of the gas double from its original value?
(Multiple Choice)
4.8/5  (46)
(46)
What is the average translational kinetic energy of a nitrogen molecule in the air in a room in which the air temperature is 17°C? The Boltzmann constant is 1.38 × 10-23 J/K.
(Multiple Choice)
4.7/5  (30)
(30)
A blacksmith is flattening a steel plate having dimensions 10 cm × 15 cm × 1 mm. He has heated the plate to 900 K. If the emissivity of the plate is 0.75, at what rate does it lose energy by radiation? Ignore any heat exchange with the surroundings. (σ = 5.67 × 10-8 W/m2 ∙ K4)
(Multiple Choice)
4.9/5  (33)
(33)
In grinding a steel knife, the metal can get as hot as 400°C. If the blade has a mass of 80 g, what is the minimum amount of water needed at 20°C if the water is to remain liquid and not rise above 100°C when the hot blade is quenched in it? The specific heat of the steel is 0.11 cal/g ∙ C° and the specific heat of water is 1.0 cal/g ∙ C°.
(Multiple Choice)
4.8/5  (34)
(34)
An aluminum electric tea kettle with a mass of 500 g is heated with a 500-W heating coil. How long will it take to heat up 1.0 kg of water from 18°C to 98°C in the tea kettle? The specific heat of aluminum is 900 J/kg ∙ K and that of water is 4186 J/kg ∙ K.
(Multiple Choice)
4.9/5  (40)
(40)
A steel bridge is 1000 m long at -20°C in winter. What is the change in length when the temperature rises to 40°C in summer? The average coefficient of linear expansion of this steel is 11 × 10-6 K-1.
(Multiple Choice)
4.9/5  (40)
(40)
A solid object has a volume density ?0 at a temperature of 315 K. The coefficient of volume expansion for the material of which it is made is 7.00 × 10-5 K-1. What will be its density (in terms of ?0 at a temperature of 425 K, assuming that it does not melt and that its thermal properties do not change with temperature?
(Short Answer)
4.8/5  (31)
(31)
How many moles are there in 2.00 kg of copper? The atomic weight of copper is 63.5 g/mol and its density is 8.90 g/cm3.
(Multiple Choice)
4.8/5  (41)
(41)
A compression at a constant pressure of 200 kPa is performed on 8.00 moles of an ideal monatomic gas. The compression reduces the volume of the gas from to How much work was done by the gas during this process?
(Multiple Choice)
4.8/5  (36)
(36)
If 150 kcal of heat raises the temperature of 2.0 kg of a material by 400 F°, what is the specific heat capacity of the material?
(Multiple Choice)
4.9/5  (30)
(30)
An ideal gas is compressed isobarically to one-third of its initial volume. The resulting pressure will be
(Multiple Choice)
4.8/5  (31)
(31)
Showing 41 - 60 of 220
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)