Exam 12: Temperature and Heat
Exam 1: Introduction and Mathematical Concepts67 Questions
Exam 2: Kinematics in One Dimension103 Questions
Exam 3: Kinematics in Two Dimensions68 Questions
Exam 4: Forces and Newtons Laws of Motion103 Questions
Exam 5: Dynamics of Uniform Circular Motion59 Questions
Exam 6: Work and Energy78 Questions
Exam 7: Impulse and Momentum65 Questions
Exam 8: Rotational Kinematics55 Questions
Exam 9: Rotational Dynamics57 Questions
Exam 10: Simple Harmonic Motion and Elasticity63 Questions
Exam 11: Fluids65 Questions
Exam 12: Temperature and Heat66 Questions
Exam 13: The Transfer of Heat42 Questions
Exam 14: The Ideal Gas Law and Kinetic Theory55 Questions
Exam 15: Thermodynamics79 Questions
Exam 16: Waves and Sound67 Questions
Exam 17: The Principle of Linear Superposition and Interference Phenomena51 Questions
Exam 18: Electric Forces and Electric Fields61 Questions
Exam 19: Electric Potential Energy and the Electric Potential70 Questions
Exam 20: Electric Circuits99 Questions
Exam 21: Magnetic Forces and Magnetic Fields66 Questions
Exam 22: Electromagnetic Induction71 Questions
Exam 23: Alternating Current Circuits84 Questions
Exam 24: Electromagnetic Waves66 Questions
Exam 25: The Reflection of Light: Mirrors42 Questions
Exam 26: The Refraction of Light: Lenses and Optical Instruments102 Questions
Exam 27: Interference and the Wave Nature of Light57 Questions
Exam 28: Special Relativity62 Questions
Exam 29: Particles and Waves54 Questions
Exam 30: The Nature of the Atom73 Questions
Exam 31: Nuclear Physics and Radioactivity33 Questions
Exam 32: Ionizing Radiation, Nuclear Energy, and Elementary Particles43 Questions
Select questions type
The coefficient of linear expansion of a certain solid is 11 × 10-6/C°.Assuming this solid behaves like most solids, what is its coefficient of volume expansion?
(Multiple Choice)
4.8/5  (36)
(36)
Heat is added to a substance, but its temperature does not rise.Which one of the following statements provides the best explanation for this observation?
(Multiple Choice)
4.9/5  (45)
(45)
Given the following information, determine the relative humidity at 15 °C. 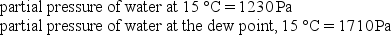
(Multiple Choice)
4.9/5  (40)
(40)
The units of heat are equivalent to those of which one of the following quantities?
(Multiple Choice)
4.9/5  (31)
(31)
An aluminum plate has a length of 0.12 m and a width of 0.10 m at 25 °C.The plate is uniformly heated to 225 °C.If the linear expansion coefficient for aluminum is 2.3 × 10-5/C°, what is the change in the area of the plate as a result of the increase in temperature?
(Multiple Choice)
4.8/5  (39)
(39)
Heat is added to a sample of water in an insulated container.Which one of the following statements is necessarily true?
(Multiple Choice)
4.9/5  (45)
(45)
What is the specific heat capacity of this substance in its solid state?
(Multiple Choice)
4.9/5  (32)
(32)
A 2.00-kg metal object requires 1.00 × 104 J of heat to raise its temperature from 20.0 °C to 40.0 °C.What is the specific heat capacity of the metal?
(Multiple Choice)
4.7/5  (46)
(46)
Which one of the following temperatures is approximately equal to the typical temperature of a classroom?
(Multiple Choice)
4.8/5  (44)
(44)
The specific heat capacity of iron is approximately half that of aluminum.Two balls of equal mass, one made of iron and the other of aluminum, both at 90 °C, are dropped into a thermally insulated jar that contains an equal mass of water at 25 °C.Thermal equilibrium is eventually reached.Which one of the following statements concerning the final temperatures is true?
(Multiple Choice)
4.8/5  (38)
(38)
During an evening news broadcast in Helsinki, Finland, the meteorologist indicated that the day's lowest temperature was -6.0 °C.What is the corresponding value of this temperature on the Fahrenheit scale?
(Multiple Choice)
4.9/5  (35)
(35)
A 2.0-g sample of steam at 100 °C loses 1140 calories of heat.What is the resulting temperature of the sample?
(Multiple Choice)
4.9/5  (33)
(33)
A 200.0-kg object is attached via an ideal pulley system to paddle wheels that are submerged in 0.480 kg of glycerin at 20.0 °C in an insulated container as shown.Then, the object falls through a distance of 5.00 m causing the paddle wheel to turn.Assuming all of the mechanical energy lost by the falling object goes into the water, determine the final temperature of the glycerin.The specific heat capacity of glycerin is 2410 J/ 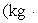 Co).
Co). 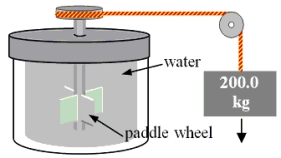
(Multiple Choice)
4.8/5  (33)
(33)
What is the specific heat capacity of this substance in its liquid state?
(Multiple Choice)
4.9/5  (30)
(30)
12-1
A 2.00-kg metal block slides on a rough, horizontal surface inside an insulated pipe.After sliding a distance of 500.0 m, its temperature is increased by 2.00 °C.Note: Assume that all of the heat generated by frictional heating goes into the metal block.For this metal, the specific heat capacity is 0.150 cal/(g · C°).  -Complete the following statement: When a substance undergoes fusion it
-Complete the following statement: When a substance undergoes fusion it
(Multiple Choice)
4.9/5  (42)
(42)
Which one of the following would probably not be used as a temperature sensitive property in the construction of a thermometer?
(Multiple Choice)
4.8/5  (38)
(38)
What is the minimum amount of energy required to completely melt a 7.25-kg lead brick which has a starting temperature of 18.0 °C? The melting point of lead is 328 °C.The specific heat capacity of lead is 128 J/(kg · C°); and its latent heat of fusion is 23 200 J/kg.
(Multiple Choice)
4.9/5  (34)
(34)
An ordinary mercury thermometer at room temperature is quickly placed in a beaker of hot water.The mercury column is observed to drop slightly before it rises to the final equilibrium temperature.Which one of the following statements is the best explanation for this behavior?
(Multiple Choice)
4.7/5  (36)
(36)
A thin, circular disc is made of lead and has a radius of 0.0350 cm at 20.0 °C.Determine the change in the area of the circle if the temperature is increased to 625.0 °C.The coefficient of linear thermal expansion for lead is 29.0 × 10-6/C°.
(Multiple Choice)
4.8/5  (32)
(32)
Complete the following statement: Bimetallic strips used as adjustable switches in electric appliances consist of metallic strips that must have different
(Multiple Choice)
4.9/5  (40)
(40)
Showing 21 - 40 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)