Exam 44: Nuclear Structure
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
Rutherford's experiment, in which he fired alpha particles of 7.7 MeV kinetic energy at a thin gold foil, showed that nuclei were very much smaller than the size of an atom because
(Multiple Choice)
4.9/5  (36)
(36)
One of the naturally occurring radioactive series begins with 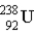 . Which of the following isotopes is the stable isotope at the end of this series?
. Which of the following isotopes is the stable isotope at the end of this series?
(Multiple Choice)
4.7/5  (32)
(32)
The chart below shows part of the radioactive series beginning with the isotope  . The isotope marked with an X is
. The isotope marked with an X is 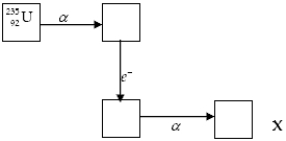
(Multiple Choice)
4.8/5  (35)
(35)
Radioactive technetium, a gamma emitter, is taken up by the heart muscle in a medical test. The detector for the radiation emitted from the heart could be a
(Multiple Choice)
4.8/5  (24)
(24)
Which of the effects listed below is not a major effect influencing the binding energy of the nucleus in the liquid-drop model?
(Multiple Choice)
4.8/5  (41)
(41)
Two nuclei may have equal Z, but different A, because they contain
(Multiple Choice)
4.8/5  (37)
(37)
How can a nucleus be described by particular values of A, Z and N when the mass of the nucleus is not equal to Zmp + Nmn, where mp and mn are the masses of free protons and neutrons?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 81 - 89 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)