Exam 44: Nuclear Structure
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
When a beam of nuclear radiation of initial intensity I0 passes through a thickness x of material, the intensity of the beam exiting the material is I =
(Multiple Choice)
4.9/5  (33)
(33)
The nuclear probability of interacting with neutrons depends most strongly on the neutron's
(Multiple Choice)
4.9/5  (41)
(41)
A neutron is characterized by the term "thermal neutron" when
(Multiple Choice)
4.8/5  (35)
(35)
44 g of petrified wood was found in a petrified forest. A sample showed a 14C activity of 100 decays/minute. How long has the tree been dead (in years)? (The half-life of carbon-14 is 5730 years and freshly cut wood contains 6.5 × 1010 atoms of 14C per gram.)
(Multiple Choice)
4.8/5  (35)
(35)
A glass container holds equal numbers of atoms of phosphorus 30 with a half-life of 2.5 minutes and of nitrogen 13 with a half-life of 10 minutes. After 20 minutes the ratio of the number of nitrogen atoms remaining to the number of phosphorus atoms remaining is
(Multiple Choice)
4.8/5  (40)
(40)
Background radiation from cosmic rays and radioactive nuclei in our surroundings is about 0.13 rem/year. Suppose we assume this all comes from cosmic rays which have an RBE factor of 1.0. The RBE factor for the most dangerous types of radiation is 20. How many rads of the most dangerous radiation could a 100-year-old person have been exposed to in her lifetime without having gone over the recommended limit of 0.5 rem/year?
(Multiple Choice)
4.8/5  (31)
(31)
A principal mechanism for energy loss during nuclear fusion is bremsstrahlung. This loss is associated with
(Multiple Choice)
4.9/5  (37)
(37)
How much energy (in MeV) is released when a 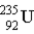 fissions to
fissions to 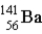 and
and 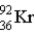 in the reaction
in the reaction 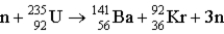 m(n) = 1.008665 u m(U) = 235.043915 u
M(Ba) = 140.9139 u
M(Kr) = 91.8973 u
U = 1.66 × 10−27 kg
m(n) = 1.008665 u m(U) = 235.043915 u
M(Ba) = 140.9139 u
M(Kr) = 91.8973 u
U = 1.66 × 10−27 kg
(Multiple Choice)
4.7/5  (31)
(31)
The nuclear reaction(s) that is(are) most likely to be employed in fusion reactors on Earth is(are)
(Multiple Choice)
4.8/5  (34)
(34)
A neutron is known to undergo beta decay (n → p + e− + 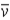 ). A reasonable mean lifetime for free neutrons is
). A reasonable mean lifetime for free neutrons is
(Multiple Choice)
4.9/5  (46)
(46)
When a fast neutron collides with a hydrogen or deuterium nucleus, the most likely result is that
(Multiple Choice)
4.8/5  (48)
(48)
Calculate the binding energy per nucleon (MeV/nucleon) for tritium, ( 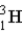 ) a radioactive isotope of hydrogen. Assume:
M p = 1.007 825 u
M n = 1.008 665 u
M t = 3.016 05 u
U = 1.66 × 10−27 kg
) a radioactive isotope of hydrogen. Assume:
M p = 1.007 825 u
M n = 1.008 665 u
M t = 3.016 05 u
U = 1.66 × 10−27 kg
(Multiple Choice)
5.0/5  (26)
(26)
How many radioactive atoms are present in a sample that has an activity of 0.5 μCi and a half-life of 10 years? (1 curie = 3.7 × 1010 decays/s)
(Multiple Choice)
4.7/5  (33)
(33)
When a neutron decays, a proton and an electron are observed. When the electrons emitted from a sample of neutrons are observed, they are found to have different kinetic energies. This was accounted for by
(Multiple Choice)
4.9/5  (34)
(34)
It is often possible to use atomic masses when calculating the binding energy of a nucleus. This is not true for calculating the Q value for the e+ decay process since
(Multiple Choice)
4.8/5  (34)
(34)
The theory of nuclear astrophysics is that all the heavy elements like uranium are formed in the interior of massive stars. These stars eventually explode, releasing these elements into space. If we assume that at the time of the explosion there were equal amount of U-235 and U-238, how long ago did the star(s) explode that released the elements that formed our Earth? The present U-235/U-238 ratio is 0.0070. [The half-lives of U-235 and U-238 are 0.7 × 109 yr and 4.47 × 109 yr.]
(Short Answer)
4.8/5  (42)
(42)
What value of Z (atomic number) and A (mass number) result in the following alpha decay? 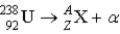
(Multiple Choice)
4.9/5  (29)
(29)
Showing 21 - 40 of 89
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)