Exam 42: Atomic Physics
Exam 1: Physics and Measurement25 Questions
Exam 2: Motion in One Dimension66 Questions
Exam 3: Vectors47 Questions
Exam 4: Motion in Two Dimensions79 Questions
Exam 5: The Laws of Motion113 Questions
Exam 6: Circular Motion and Other Applications of Newtons Laws55 Questions
Exam 7: Energy of a System74 Questions
Exam 8: Conservation of Energy84 Questions
Exam 9: Linear Momentum and Collisions89 Questions
Exam 10: Rotation of a Rigid Object About a Fixed Axis82 Questions
Exam 11: Angular Momentum46 Questions
Exam 12: Static Equilibrium and Elasticity34 Questions
Exam 13: Universal Gravitation47 Questions
Exam 14: Fluid Mechanics53 Questions
Exam 15: Oscillatory Motion41 Questions
Exam 16: Wave Motion82 Questions
Exam 18: Superposition and Standing Waves72 Questions
Exam 19: Temperature47 Questions
Exam 20: The First Law of Thermodynamics61 Questions
Exam 21: The Kinetic Theory of Gases38 Questions
Exam 22: Heat Engines, Entropy, and the Second Law of Thermodynamics55 Questions
Exam 23: Electric Fields67 Questions
Exam 24: Gausss Law82 Questions
Exam 25: Electric Potential111 Questions
Exam 26: Capacitance and Dielectrics63 Questions
Exam 27: Current and Resistance34 Questions
Exam 28: Direct-Current Circuits84 Questions
Exam 29: Magnetic Fields80 Questions
Exam 30: Sources of the Magnetic Field95 Questions
Exam 31: Faradays Law62 Questions
Exam 32: Inductance23 Questions
Exam 33: Alternating-Current Circuits65 Questions
Exam 34: Electromagnetic Waves40 Questions
Exam 35: The Nature of Light and the Principles of Ray Optics37 Questions
Exam 36: Image Formation43 Questions
Exam 37: Wave Optics48 Questions
Exam 38: Diffraction Patterns and Polarization47 Questions
Exam 39: Relativity34 Questions
Exam 40: Introduction to Quantum Physics48 Questions
Exam 41: Quantum Mechanics33 Questions
Exam 42: Atomic Physics59 Questions
Exam 43: Molecules and Solids46 Questions
Exam 44: Nuclear Structure89 Questions
Exam 46: Particle Physics and Cosmology34 Questions
Select questions type
Quantum physics agrees with the classical physics limit when
(Multiple Choice)
4.7/5  (38)
(38)
The s, p, d, f, symbols represent values of the quantum number
(Multiple Choice)
4.9/5  (40)
(40)
One of the main problems with the Bohr model of the hydrogen atom when compared with the results of the methods of quantum mechanics used to describe atoms, was that the Bohr model predicted
(Multiple Choice)
4.9/5  (36)
(36)
Adam and Eve are contemplating the beauty of the hydrogen atom. Adam claims that the quantum states with a given value of the principal quantum number n can have any value of the orbital quantum number 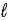 . Eve says that the Snake told her that a state with a given value of
. Eve says that the Snake told her that a state with a given value of 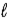 could have any value of n. Which one, if either, is correct, and why?
could have any value of n. Which one, if either, is correct, and why?
(Multiple Choice)
4.8/5  (39)
(39)
The energy difference between the upper and lower levels in a certain laser is 1.9593 eV. What is the wavelength of the light emitted by the laser?
(Short Answer)
4.9/5  (39)
(39)
A Li2+ ion undergoes a transition from the n = 4 to the n = 3 state. The energy of the emitted photon is
(Multiple Choice)
4.7/5  (43)
(43)
In the Bohr model of the hydrogen atom, the total energy of the electron-proton system is
(Multiple Choice)
4.8/5  (37)
(37)
If P(r) is the radial probability density function for an electron in the ground state of a hydrogen atom, the most probable value for r can be found from
(Multiple Choice)
4.9/5  (31)
(31)
Light is emitted by hydrogen atoms in the visible range for a hydrogen atom. Its wavelength is 656 nm. What value of n is associated with the light? (RH = 1.097 × 107 m−1)
(Multiple Choice)
4.8/5  (35)
(35)
The number of states in the He+ ion corresponding to the principle quantum number n = 5 are
(Multiple Choice)
4.9/5  (37)
(37)
The radial portion of the de Broglie wavefunction for an electron in the ground state of the hydrogen atom is Ψ1s(r) = 1/( 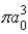 )1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
)1/2 exp(−r/a0) where a0 is the Bohr radius. The probability of finding the electron is
(Multiple Choice)
4.9/5  (32)
(32)
In the subshell of the Li2+ ion with orbital quantum number 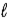 , the allowed values of the magnetic quantum number
, the allowed values of the magnetic quantum number 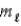 are
are
(Multiple Choice)
4.9/5  (36)
(36)
A hydrogen atom emits a photon of wavelength 657.7 nm. From what energy state to what lower energy state did the electron jump?
(Short Answer)
4.8/5  (42)
(42)
An electron in a hydrogen atom makes a transition from the n = 3 to the n = 1 energy state. Determine the wavelength of the emitted photon (in nm).
(Multiple Choice)
4.9/5  (36)
(36)
Characteristic x-rays can be produced by bombarding targets with electrons. These x-rays occur when
(Multiple Choice)
4.9/5  (28)
(28)
Suppose a beam of electrons is incident on a collection of hydrogen atoms, all of which are in the lowest energy state (n = 1). What is the minimum energy the electrons can have if they are to excite the hydrogen atoms into the n = 2 state?
(Short Answer)
4.9/5  (41)
(41)
A hydrogen atom is in its first excited state (n = 2). The linear momentum of the electron is (in kg ⋅ m/s)
(Multiple Choice)
4.8/5  (41)
(41)
Showing 21 - 40 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)