Exam 8: The Quantum Mechanical Atom
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
All orbitals with the same value of the principal quantum number and the secondary quantum number are said to belong to the same
(Multiple Choice)
4.8/5  (37)
(37)
What is the energy of one mole of photons of visible light having a wavelength of 4.89 × 102 nm?
(Multiple Choice)
4.8/5  (38)
(38)
Calculate the wavelength of the spectral line in the spectrum of hydrogen for which n1 = 1 and n2 = 3.Hint: Use the Rydberg equation to solve.
(Multiple Choice)
4.8/5  (37)
(37)
The lowest energy state of the hydrogen atom is the most stable one, and occurs when its electron has n = 1, which brings the electron closest to the nucleus.
(True/False)
4.9/5  (28)
(28)
What is the energy of one mole of photons whose wavelength is 5.461 × 102 nm?Hint: Pay careful attention to your units. 1 m = 109 nm.
(Multiple Choice)
4.9/5  (41)
(41)
A lamp that generates 25 W of energy (1 W = 1 J·s-1)emits 5.5 × 1019 photons of lightin 1 s. Calculate the wavelength (in nm)of the emitted light.Hint: Use your energy in watts and convert to J/s.
(Short Answer)
4.9/5  (34)
(34)
Calculate the number of moles of photons required to heat 245 mL of water from 22.5°C to its boiling temperature in a microwave oven, which operates at a frequency of 12.50 cm. Assume the density of water is 1.0 g/mL and the specific heat capacity of water is 4.184 J/g ? °C.Hint: Water boils at 100 °C. Watch your units when solving this problem.
(Multiple Choice)
4.9/5  (33)
(33)
Based on its expected electron configuration, element Z = 120
(Multiple Choice)
4.9/5  (36)
(36)
A correct description for the electron configuration of a selenium atom is
(Multiple Choice)
5.0/5  (34)
(34)
The lowest energy state of an atom is the most stable one and is called the ________.
(Short Answer)
4.9/5  (37)
(37)
Calculate the wavelength, in meters, of an electron (mass = 9.109 × 10-28
(Short Answer)
4.9/5  (41)
(41)
The element ________ has the ground state configuration of 1s2 2s2 2p6 3s1.
(Short Answer)
4.9/5  (42)
(42)
Which atom in the set [Sr, Fr, Hg, Ga, Cr, Sn] has the smallest first ionization energy?
(Short Answer)
4.9/5  (35)
(35)
One way in which very small particles display wavelike behavior is their ability to be diffracted when they are in beams.
(True/False)
5.0/5  (35)
(35)
Which of the following gives a possible quantum number assignment for the last electron added to the oxygen atom when developing the electron configuration using the aufbau principle? 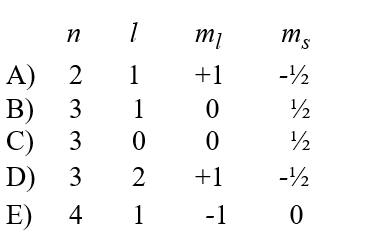
(Short Answer)
4.8/5  (35)
(35)
According to the photoelectric effect, increasing the intensity of light will always increase the number of electrons that escape the surface of a material.
(True/False)
5.0/5  (35)
(35)
Showing 181 - 200 of 219
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)