Exam 8: The Quantum Mechanical Atom
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
A correct description for the electron configuration of an iron atom is
(Multiple Choice)
4.8/5  (40)
(40)
The frequency of a wave is related to the energy of the radiation.
(True/False)
4.8/5  (37)
(37)
An electron behaves as if it were spread out in space around the nucleus in a sort of ________.
(Short Answer)
4.8/5  (40)
(40)
The definite energies associated with specific wavelengths in the emission spectrum of atomic hydrogen suggest that
(Multiple Choice)
4.9/5  (38)
(38)
Generally speaking, both anions and cations have larger radii than their parent atoms.
(True/False)
4.8/5  (39)
(39)
Which one of the following types of radiation has the lowest frequency?
(Multiple Choice)
4.8/5  (42)
(42)
Calculate the frequency of visible light having a wavelength of 589.3 nm.
(Multiple Choice)
4.8/5  (33)
(33)
Calculate the wavelength of the transition from n = 2 to n = 3 for an ion with a single electron, where the charge on the ion is +2.Hint: Use the Rydberg equation when calculating transition wavelength.
(Short Answer)
4.9/5  (34)
(34)
What is the wavelength of radiation which has an energy of 216.1 kJ per mole of photons?
(Multiple Choice)
4.7/5  (35)
(35)
An electron with an n = 2 quantum number may also have a quantum number l = 2.
(True/False)
5.0/5  (38)
(38)
In the Rydberg equation, if n1 = 1, an acceptable value of n2 is ________.
(Short Answer)
4.9/5  (44)
(44)
Which placement of electrons is never encountered in the ground state configuration of an atom?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following gives a possible quantum number assignment for the last electron added to the sodium atom when developing the electron configuration using the aufbau principle? 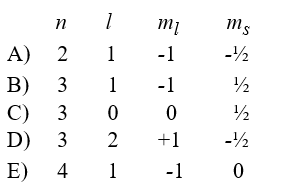
(Short Answer)
4.9/5  (32)
(32)
The maximum number of electrons in an atom that can have the following exact same set of quantum numbers is ________. 
(Short Answer)
4.8/5  (40)
(40)
Showing 21 - 40 of 219
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)