Exam 8: The Quantum Mechanical Atom
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
The operation of the electron microscope is based on the particle nature of the electron.
(True/False)
4.8/5  (44)
(44)
The uncertainty principle explains the fact that certain atoms, for instance silver, have different electronic configurations in their ground states than predicted by the aufbau principle.
(True/False)
4.8/5  (40)
(40)
The decrease in atomic radius as one progresses from element Z = 11 to Z = 18 in the periodic table can be attributed to
(Multiple Choice)
4.9/5  (35)
(35)
Which atom in the set [Ba, Cr, N, Sn, Mg, Se] would you expect to have the largest atomic radius?
(Short Answer)
4.7/5  (45)
(45)
The electron microscope makes use of the waves associated with very slow moving electrons in order to observe small objects.
(True/False)
4.8/5  (36)
(36)
Which atom in the set [Y, Cr, Mg, N, Ba, As, Sn] has the largest first ionization energy?
(Short Answer)
4.8/5  (43)
(43)
What is the energy required to excite a hydrogen atom by causing an electronic transition from the n = 2 to the n = 5 energy level? Recall that the quantized energies of the levels in the hydrogen atom are given by: 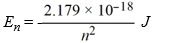
(Short Answer)
4.9/5  (35)
(35)
A particular energy level in an atom has a value of 3 for the secondary quantum number. What is the maximum number of electrons that can occupy this energy level?
(Short Answer)
4.7/5  (37)
(37)
The letter designation for the subshell in an atom is based on
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following are expected to be diamagnetic in their ground state electron configurations: S, Xe, Hg, P, Br?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following choices represent an electron configuration for an oxygen atom in an excited state? Hint: What is the difference between ground state and excited state?
(Multiple Choice)
4.7/5  (48)
(48)
The structure of the periodic table can be correlated with the electron configuration of the elements.
(True/False)
4.9/5  (36)
(36)
The last electron of the aluminum atom is placed in the 3p orbital of lowest energy before placing an electron in any other 3p orbital.
(True/False)
4.8/5  (48)
(48)
How many pairs of electrons are present in the 3d subshell in the ground state electron configuration of the Cu atom?
(Multiple Choice)
4.7/5  (38)
(38)
A possible set of quantum numbers for an electron in the partially filled subshell in a gallium atom in its ground state configuration would be 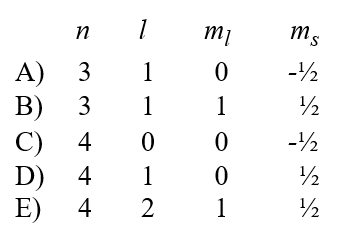
(Short Answer)
4.8/5  (35)
(35)
Showing 101 - 120 of 219
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)