Exam 8: The Quantum Mechanical Atom
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
An electron in an atom can be described by solutions to the wave equation. The solutions are identified by quantum numbers. How many quantum numbers are required for describing one electron in the atom?
(Short Answer)
4.8/5  (45)
(45)
Based on the order in which the subshells are filled and the electron configuration of atoms, element Z = 111
(Multiple Choice)
4.8/5  (38)
(38)
What is the wavelength of electromagnetic radiation which has a frequency of 7.215 × 1016 s-1 (c = 2.998 × 108 m/s)?
(Short Answer)
5.0/5  (44)
(44)
Which of the following metals are expected to be paramagnetic in their ground state electron configurations: Ca, Ti, Cd, Cu, Al? Hint: Look for unpaired electrons.
(Multiple Choice)
4.7/5  (32)
(32)
The requirement that the ground state configuration of an atom is generated by placing electrons into orbitals from the lowest energy to the highest energy, observing the maximum number allowed for each of these levels is
(Multiple Choice)
4.8/5  (42)
(42)
A line in the Balmer series of the hydrogen emission spectrum occurs at 397 nm. Calculate the value of n for the Bohr orbit involved in the emission of this light.Hint: The Balmer series
(Short Answer)
4.8/5  (42)
(42)
A possible set of quantum numbers for an electron in the partially filled subshell in a potassium atom in its ground state configuration would be 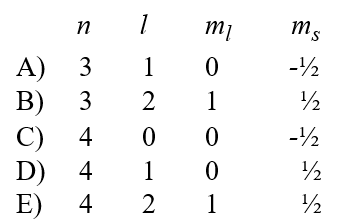
(Short Answer)
4.9/5  (43)
(43)
In the orbital diagram for carbon in the ground state, the sixth electron cannot be placed in any one of the 2p orbitals because it must be placed in an empty orbital.
(True/False)
4.8/5  (39)
(39)
All orbitals with the same value of the principal quantum number are said to belong to the same
(Multiple Choice)
4.8/5  (39)
(39)
Electromagnetic radiation comes in a broad range of frequencies called the ________.
(Short Answer)
4.9/5  (38)
(38)
An unidentified element is known to have an electron configuration, [X] ns2, in its ground state. This element must be in the same family as
(Multiple Choice)
4.8/5  (32)
(32)
A continuous spectrum contains a series of brightly colored lines observed against a dark background.
(True/False)
4.9/5  (30)
(30)
What is the wavelength of electromagnetic radiation which has a frequency of 3.818 × 1014 s-1?
(Multiple Choice)
4.9/5  (39)
(39)
The first description of the electron in the hydrogen atom by application of the wave nature of matter was presented by
(Multiple Choice)
4.8/5  (40)
(40)
Which placement of electrons is never encountered in the ground state configuration of an atom?
(Multiple Choice)
4.8/5  (35)
(35)
Showing 81 - 100 of 219
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)