Exam 8: The Quantum Mechanical Atom
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 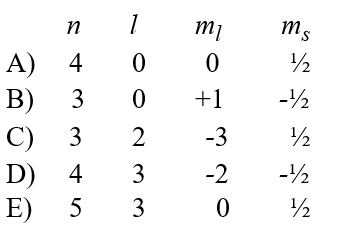
(Short Answer)
4.9/5  (35)
(35)
There are more unpaired electrons in the ground state electron configuration of a chromium atom than of a manganese atom.
(True/False)
5.0/5  (42)
(42)
Which atom in the set [O, F, Ne, Ar, Cl, K, Ga] has the greatest electron affinity?
(Short Answer)
4.8/5  (35)
(35)
What is the maximum number of electrons that can fill all the orbitals of a d subshell?
(Multiple Choice)
4.8/5  (43)
(43)
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 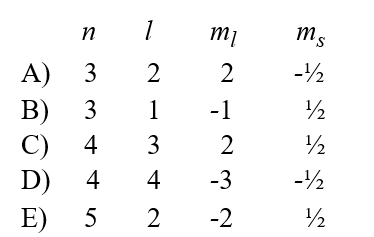
(Short Answer)
4.7/5  (43)
(43)
Calculate the wavelength of the spectral line in the spectrum of hydrogen for which n1 = 4 and n2 = 7.Hint: Use the Rydberg equation to solve.
(Multiple Choice)
4.9/5  (36)
(36)
A good rule of thumb is: the higher the atomic number, the greater the electronegativity of the atom.
(True/False)
4.9/5  (33)
(33)
Which statement is true concerning Bohr's model of the atom?
(Multiple Choice)
4.9/5  (37)
(37)
Calculate the wavelength of an electron (mass = 9.109 × 10-31 kg)traveling at4.38 × 106 m/s.
(Multiple Choice)
4.8/5  (33)
(33)
How many electrons can be placed in a shell in which the principal quantum number, n = 3?
(Multiple Choice)
4.8/5  (34)
(34)
The amplitude of a wave at any given point is related to the probability of finding the electron at that location.
(True/False)
5.0/5  (38)
(38)
The electron microscope makes use of the waves associated with fast moving electrons in order to observe small objects.
(True/False)
4.8/5  (38)
(38)
A correct description for the electron configuration of a chromium atom is
(Multiple Choice)
4.7/5  (44)
(44)
The probability of finding the electron varies in space, and can be represented by the square of the ________.
(Short Answer)
4.8/5  (39)
(39)
Which placement of electrons is never encountered in the ground state configuration of an atom?
(Multiple Choice)
4.8/5  (34)
(34)
Which of the following choices is the correct electron configuration for a cobalt atom? 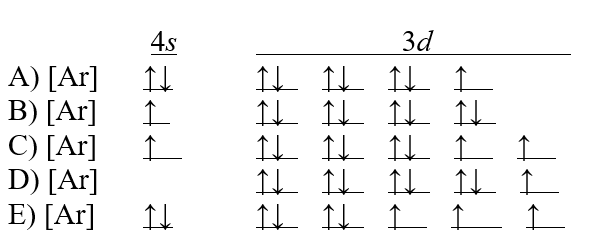
(Short Answer)
4.8/5  (39)
(39)
Given the following sets of quantum numbers for n, l, ml, and ms, which one of these sets is not possible for an electron in an atom? 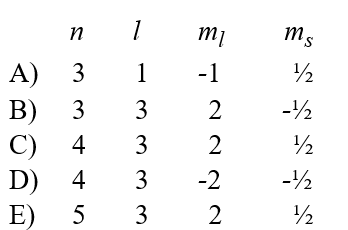
(Short Answer)
4.9/5  (39)
(39)
In the orbital diagram for boron, the fifth electron can be placed in any one of the 2p orbitals, because they are all equal in ________.
(Short Answer)
4.8/5  (45)
(45)
Showing 201 - 219 of 219
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)