Exam 8: The Quantum Mechanical Atom
Exam 1: A Very Brief History of Chemistry90 Questions
Exam 2: Scientific Measurements224 Questions
Exam 3: Elements, Compounds, and the Periodic Table227 Questions
Exam 4: The Mole and Stoichiometry207 Questions
Exam 5: Molecular View of Reactions in Aqueous Solutions237 Questions
Exam 6: Oxidation-Reduction Reactions175 Questions
Exam 7: Energy and Chemical Change176 Questions
Exam 8: The Quantum Mechanical Atom219 Questions
Exam 9: The Basics of Chemical Bonding167 Questions
Exam 10: Theories of Bonding and Structure196 Questions
Exam 11: Properties of Gases162 Questions
Exam 12: Intermolecular Attractions and the Properties of Liquids and Solids189 Questions
Exam 13: Mixtures at the Molecular Level: Properties of Solutions133 Questions
Exam 14: Chemical Kinetics151 Questions
Exam 15: Chemical Equilibrium109 Questions
Exam 16: Acids and Bases, a Molecular Look104 Questions
Exam 17: Acid-Base Equilibria in Aqueous Solutions184 Questions
Exam 18: Solubility and Simultaneous Equilibria120 Questions
Exam 19: Thermodynamics109 Questions
Exam 20: Electrochemistry143 Questions
Exam 21: Nuclear Reactions and Their Role in Chemistry115 Questions
Exam 22: Metal Complexes113 Questions
Exam 23: Organic Compounds, Polymers, and Biochemicals140 Questions
Select questions type
A police radar unit is operating on a frequency of 9.527 gigahertz. What is the wavelength of the radiation being employed?
(Multiple Choice)
4.7/5  (40)
(40)
The de Broglie relationship provides a link between which two properties of the electron?
(Multiple Choice)
4.9/5  (30)
(30)
The following are all ionization energies for beryllium. 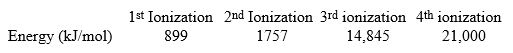 How much energy would be needed to remove three electrons from a ground state beryllium atom?
How much energy would be needed to remove three electrons from a ground state beryllium atom?
(Short Answer)
4.9/5  (34)
(34)
What is the energy of one photon of microwave radiation with a wavelength of 0.158 m?
(Multiple Choice)
4.9/5  (27)
(27)
What is the energy of one mole of photons of visible light having a wavelength of 486.1 nm?
(Multiple Choice)
4.7/5  (40)
(40)
Calculate the wavelength, in nanometers, of light emitted by a hydrogen atom when the electron falls from an n = 7 energy level to an n = 4 energy level. Recall that the quantized energies of the levels in the hydrogen atom are given by: 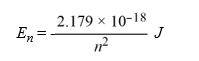
(Multiple Choice)
4.9/5  (30)
(30)
A possible set of quantum numbers for an electron in the partially filled subshell in a vanadium atom in its ground state configuration would be 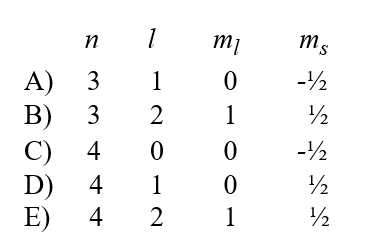
(Short Answer)
4.8/5  (34)
(34)
The diffraction of electrons is the principle on which the electron microscope is based.
(True/False)
4.8/5  (36)
(36)
There are several possible arrangements of electrons when you try to place 7 electrons in a 3d subshell. To determine the correct distribution for the ground state we are guided by
(Multiple Choice)
4.8/5  (38)
(38)
Kevin is working on a summer project in which he is using a tunable dye laser. At first, he used radiation on a wavelength of 488.5 nm. After two weeks, his research advisor told him to increase the energy by another 55.0 kJ per mole and repeat the radiation effect tests. What wavelength should he then use? (Use c = 2.998 × 108 m/s)Hint: Account for the added energy in your formula.
(Short Answer)
4.8/5  (34)
(34)
How many unpaired electrons are in the ground state electron configuration for an iron atom?
(Short Answer)
4.9/5  (33)
(33)
The ground state electron configuration, using abbreviated electron configuration, for arsenic is ________.
(Short Answer)
4.7/5  (38)
(38)
The increase in atomic radius as one progresses within the alkali metal family from element Z = 3 to Z = 87 in the periodic table can be attributed to
(Multiple Choice)
4.8/5  (38)
(38)
The directions of maximum electron density of the three p orbitals are mutually perpendicular to each other.
(True/False)
4.8/5  (31)
(31)
Showing 141 - 160 of 219
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)