Exam 19: The Transition Metals
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
What differences might you expect between the two complexes,
Fe(CN)64- and FeF64-?(If needed, use the following equation:Spectrochemical Series I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
(Multiple Choice)
4.8/5  (40)
(40)
Aqueous copper(I) chloride is nearly colourless whereas aqueous copper(II) chloride is blue in colour. This difference is because(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
(Multiple Choice)
4.8/5  (35)
(35)
Which of the following transition metals in the 3d series has the most variability in preferred oxidation number?
(Multiple Choice)
4.8/5  (39)
(39)
[Co(NH3)5NO2]Cl2 and [Co(NH3)5ONO]Cl2 are examples of what type of isomer?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
(Multiple Choice)
4.8/5  (37)
(37)
What is the oxidation state of iron in deoxyhaemoglobin and oxyhaemoglobin?
(Multiple Choice)
4.7/5  (40)
(40)
What is the correct arrangement of Hf, Ti, and Zr in order of increasing melting point?
(Multiple Choice)
4.9/5  (32)
(32)
Which of the metals is most likely to be found in elemental form in nature?
(Multiple Choice)
4.8/5  (38)
(38)
What is the layer above molten iron in a blast furnace called?
(Multiple Choice)
4.9/5  (40)
(40)
The complex Fe(C2O4)33- has one unpaired electron. What is the electron configuration of this complex?(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
(Multiple Choice)
4.9/5  (27)
(27)
What is the correct arrangement of Cr, Ni, and Zn in order of decreasing density?
(Multiple Choice)
4.9/5  (44)
(44)
Which transition metal compounds are most likely to found as pure elements?
(Multiple Choice)
4.8/5  (30)
(30)
What is the oxidation state of rhodium in pentaamminebromorhodium bromide, [Rh(NH3)5Br]Br2?
(Multiple Choice)
5.0/5  (33)
(33)
Which of the following five complexes are paramagnetic with 2 unpaired electrons? 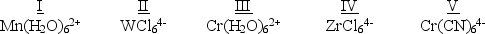 (If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
(If needed, use the following equation:Spectrochemical Series
I- < Br- < Cl- < F- < OH- < H2O< NH3 < en < NO2- < CN- < CO)
(Multiple Choice)
4.9/5  (33)
(33)
Which is the chemical formula for the compound named hexamminecobalt(III) sulphate?
(Multiple Choice)
4.7/5  (38)
(38)
The transition metals with the electron configurations of 4s23d2 and 5s24d7 are
(Multiple Choice)
5.0/5  (39)
(39)
Which of the following groups has primary use as catalysts?
(Multiple Choice)
4.9/5  (45)
(45)
Showing 21 - 38 of 38
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)