Exam 18: Macromolecules
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Which of the following would be a suitable initiator molecule for polymerization of ethylene?
(Multiple Choice)
4.9/5  (39)
(39)
What type of bond is formed when adding DNA units onto a DNA strand?
(Multiple Choice)
4.9/5  (41)
(41)
Draw the structure of the nucleotide formed from the base adenine, ribose and phosphate shown below: 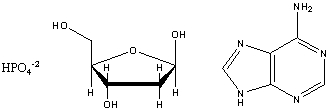
(Essay)
4.7/5  (35)
(35)
Draw the structure of the nucleotide formed from the base thymine, ribose and phosphate shown below: 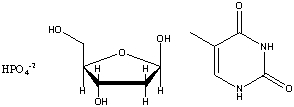
(Essay)
4.8/5  (48)
(48)
A glycosidic bond between two monosaccharides results in the formation of what two molecules?
(Multiple Choice)
4.8/5  (40)
(40)
Draw the product of the condensation reaction between dimethylamine (HN(CH3)2) and formic acid (H-C=O)-OH) 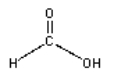
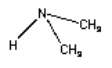 H-(C= O)-OH H-N-(CH3)2
H-(C= O)-OH H-N-(CH3)2
(Essay)
4.9/5  (48)
(48)
Draw a representative molecule containing three carbons for each of the following. 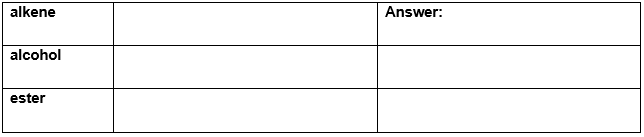
(Essay)
4.9/5  (34)
(34)
One way to synthesize polyamides is from acid chlorides and amines with the loss of HCl. Draw the structure of the combination of two units of each of the following monomers: 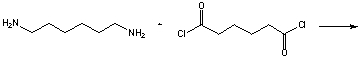
(Essay)
4.8/5  (31)
(31)
How would a scientist make a co-polymer via radical polymerization out of two monomer units with a ratio of 3 to 1? Would the scientist be able to control the order of the addition of monomer units? That is, could the scientist guarantee that the order would be A-A-A-B-A-A-A-B-A-A-A-B- and so on? If so how?
(Essay)
4.7/5  (37)
(37)
Use the following amino acids for questions
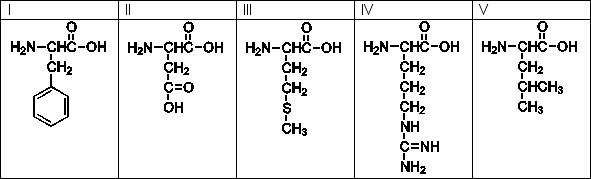 -Which of the above is the line structure for tyrosine?
-Which of the above is the line structure for tyrosine?
(Multiple Choice)
4.8/5  (39)
(39)
How do bond electrons differ from bond electrons in C == C bonds?
(Multiple Choice)
4.9/5  (38)
(38)
Use the following amino acid structures to answer questions
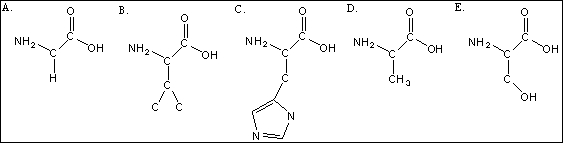 -Give the formula for amino acid shown above as B.
-Give the formula for amino acid shown above as B.
(Short Answer)
4.8/5  (36)
(36)
Complete the picture of ? -glucose where the information for carbons 1 and 5 have been left off. 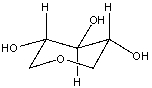
(Essay)
4.9/5  (39)
(39)
Primary, secondary and tertiary structure of proteins is determined by
(Multiple Choice)
4.7/5  (32)
(32)
Recognize and describe some properties of plastics, fibres, and elastomers.
(Essay)
4.8/5  (45)
(45)
If the primary structure of a segment of RNA is UUGCAUUGC, what sequence of DNA was this transcribed from?
(Short Answer)
4.8/5  (31)
(31)
Showing 61 - 80 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)