Exam 18: Macromolecules
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
What intermolecular force keeps cellulose from moving past each other?
(Multiple Choice)
4.8/5  (35)
(35)
Olestra is a new oil substitute in the market where a hexose has fatty acids attached to the sugar via glycosidic bonds. What is the maximum number of glycosidic bonds possible in a hexose?
(Multiple Choice)
4.9/5  (39)
(39)
What is typically used as an initiation step for radical polymerization?
(Multiple Choice)
4.9/5  (37)
(37)
What two functional groups are used to combine monosaccharides into polymers?
(Multiple Choice)
4.8/5  (45)
(45)
Which of the following will result in termination of polymer growth?
(Multiple Choice)
4.9/5  (43)
(43)
A polymer is being made and marketed by Cargill. This polymer is made from lactic acid, a by-product of ethanol production from corn. Draw the product of three lactic acids combined via condensation reactions. 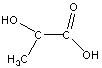
(Essay)
4.8/5  (36)
(36)
What functional groups are present in the ball and stick model shown below? 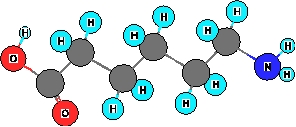 I. amine
II. Alcohol
III. carboxylic acid
IV. Ketone
V. thiol
I. amine
II. Alcohol
III. carboxylic acid
IV. Ketone
V. thiol
(Multiple Choice)
4.7/5  (44)
(44)
What is a typically termination step for radical polymerization?
(Multiple Choice)
4.8/5  (33)
(33)
What type of polymer would be best suited for an automobile bumper?
(Multiple Choice)
4.8/5  (27)
(27)
Draw the product of the condensation reaction of 1-propanol with itself. CH3CH2CH2OH.
(Essay)
4.8/5  (34)
(34)
Use the following amino acids for questions
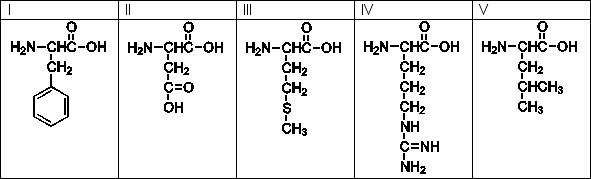 -Which of the above is the line structure for methionine?
-Which of the above is the line structure for methionine?
(Multiple Choice)
4.8/5  (33)
(33)
Draw the structures of two molecules that would form the molecule shown below in a condensation reaction. 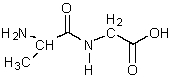
(Essay)
4.8/5  (34)
(34)
How many products containing two amino acids can be formed from the following three amino acids? 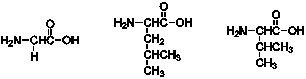
(Multiple Choice)
4.8/5  (42)
(42)
The polymer Lexan, shown below, is a condensation polymer formed with the elimination of an HCl molecule. Identify the monomers. 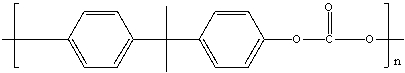
(Essay)
4.9/5  (38)
(38)
What is the line structure for the compound containing an amide-linking group? 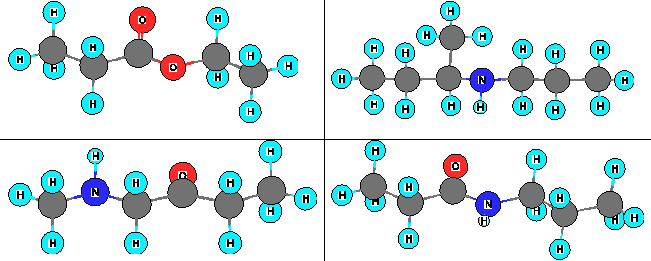
(Essay)
4.9/5  (38)
(38)
Showing 21 - 40 of 83
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)