Exam 17: Electron Transfer Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Calculate the standard free energy changes for the following redox reaction:
2 Ag+(aq) + Sn2+(aq) 2 Ag(s) + Sn4+(aq) [E°(Sn4+, 2+) = 0.151 V](If needed, refer to Table 17-1 in the text)
(Short Answer)
4.8/5  (44)
(44)
What are the possible oxidation states of corroded iron?(If needed, refer to Table 17-1 in the text)
(Multiple Choice)
4.8/5  (34)
(34)
Balance the following half reaction under neutral conditions:HSO3- SO42-
(Short Answer)
4.8/5  (45)
(45)
For the working galvanic cell shown at standard conditions, determine the balanced reaction and direction of electron flow through the wire. 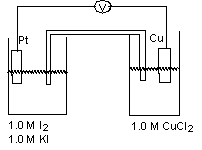 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
(Short Answer)
4.9/5  (45)
(45)
Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu2+ (aq) Zn2+ (aq) + Cu (s) E° = 1.10 VIf the cell is initially at standard conditions ([Cu2+] = [Zn2+] = 1.00 M), what are the concentrations of Cu2+ and Zn2+ when the cell potential has fallen to 1.06 V?If needed, use the following equation: G° = -nFE°, E° ![Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)](https://storage.examlex.com/TB9687/11ee726d_d433_2f97_827e_430b5f32088f_TB9687_11.jpg) , E = E° -
, E = E° - ![Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)](https://storage.examlex.com/TB9687/11ee726d_d433_2f98_827e_cfe7754dd8fc_TB9687_11.jpg) , moles e- =
, moles e- = ![Consider the Daniell cell where the cell reaction and standard potential are:Zn(s) + Cu<sup>2+</sup><sup> </sup>(aq) \rarr Zn<sup>2+</sup><sup> </sup>(aq) + Cu (s) \quad E° = 1.10 VIf the cell is initially at standard conditions ([Cu<sup>2+</sup>] = [Zn<sup>2+</sup>] = 1.00 M), what are the concentrations of Cu<sup>2+</sup><sup> </sup>and Zn<sup>2+</sup> when the cell potential has fallen to 1.06 V?If needed, use the following equation: \Delta G° = -nFE°, E° , E = E° - , moles e<sup>-</sup> = (If needed, refer to Table 17-1in the text)](https://storage.examlex.com/TB9687/11ee726d_d433_2f99_827e_3f1d17c3c852_TB9687_11.jpg) (If needed, refer to Table 17-1in the text)
(If needed, refer to Table 17-1in the text)
(Multiple Choice)
4.7/5  (47)
(47)
For the reaction given below, which half reaction occurs at the anode?
2 H2(g) + O2(g) 2 H2O(l)
(Multiple Choice)
4.7/5  (33)
(33)
Use oxidation numbers to show what is being oxidized and what is being reduced in a redox reaction.
(Essay)
4.9/5  (44)
(44)
Calculate the standard potential of the aluminium air battery in which the active materials Al(s) andO2, and the electrolyte is aqueous KOH.(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.7/5  (49)
(49)
Which of the following are redox reactions?
I. 2 N2 + 3 H2 2 NH3
II. 4 Al + 3 O2 2 Al2O3
III. 2 NO2 N2O4
IV. FeCl3 (aq) + 6 NH3(aq) (\rarr\)Fe(NH3)63+ (aq) + 3 Cl- (aq)
V. Cu2+ + Zn Cu + Zn2+
(Multiple Choice)
4.7/5  (34)
(34)
Balance the following half reaction under basic conditions:NO3- NO2-
(Short Answer)
4.9/5  (44)
(44)
Consider the redox reaction of nitric acid and copper:
Cu + HNO3 Cu(NO3)2 + NO (acidic solution)If the coefficient of Cu is 3 in the balanced equation, what is the coefficient of HNO3?
(Multiple Choice)
4.7/5  (34)
(34)
The same charge of 1.07 x 104 C is passed through three solutions: one each of Au3+, Cu+ and Pb2+ with strips of the metals as cathodes. In which cell will the greatest mass of metal be reduced and what is the mass of that metal?
(Short Answer)
4.9/5  (38)
(38)
Balance the following half reaction under acidic conditions:I2O5 I2
(Short Answer)
4.9/5  (37)
(37)
Consider the Daniell cell for which the cell reaction and standard potential are:Zn(s) + Cu2+ (aq) Zn2+ (aq) + Cu (s) E° = 1.10 VIf the cell is initially at standard conditions ([Cu2+] = [Zn2+] = 1.00 M) and assuming it contains 1 L of electrolyte, determine the mass of Zn(s) lost when the cell potential falls to 1.06 V?(If needed, refer to Table 17-1in the text)
(Short Answer)
4.9/5  (26)
(26)
Balance the reaction and calculate the standard potential for:
Zr + H2O ZrO2 + H2Given: ZrO2 + 4e- + 4 H3O+ Zr + 6 H2O (E°=-1.43 V)(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.9/5  (33)
(33)
Assign oxidation numbers to all the elements in titanium nitride, Ti3N4.
(Short Answer)
4.8/5  (36)
(36)
Calculate the equilibrium constants for the following redox reaction:
2 Cu2+ (aq) + Sn2+ (aq) 2 Cu+ (aq) + Sn4+ (aq)[E°(Sn4+, 2+) = 0.151 V] [E°(Cu2+, 1+) = 0.153 V](If needed, refer to Table 17-1 in the text)
(Short Answer)
4.9/5  (37)
(37)
Ships, storage tanks, and other large metal items may be protected from corrosion by(If needed, refer to Table 17-1in the text )
(Multiple Choice)
4.9/5  (36)
(36)
Showing 41 - 60 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)