Exam 17: Electron Transfer Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Calculate the standard potential of voltaic cells that combine the following half reactions:Pb to PbSO4 and PbO2 to PbSO4 (acid solution)(If needed, refer to Table 17-1.in the text )
(Short Answer)
4.8/5  (40)
(40)
Use the half-reaction method to balance the following redox reaction:
Cl2 Cl- + ClO- (basic solution)
(Short Answer)
4.9/5  (34)
(34)
Consider the redox process:
Cd(OH)2 + Ni(OH)2 + 2 OH- NiO2 + Cd + H2OWrite the equation for the spontaneous process and determine the free energy change for the spontaneous process. 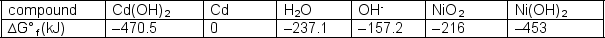
(Short Answer)
4.9/5  (46)
(46)
An electrolytic cell driving the following redox reaction has a current of 4.02 amps passed through it for 2.32 hours. How much Ag will be dissolved and how much Cu will be deposited?2 Ag (s) + Cu+2 (aq) 2 Ag+ (aq) + Cu (s)
(Short Answer)
4.8/5  (45)
(45)
For the reaction given, which half reaction occurs at the cathode?
NiO2 + Cd + H2O Cd(OH)2 + Ni(OH)2 + 2 OH-
(Multiple Choice)
4.7/5  (36)
(36)
Calculate the standard potential of the redox reaction:
2 Na + S Na2S (E° for S + 2e- = S2- = -0.508 V(If needed, refer to Table 17-1. in the text)
(Short Answer)
4.8/5  (38)
(38)
An electrochemical cell is made by immersing a piece of Cd metal into a solution of 0.100 M CdSO4 and a Zn electrode into a solution of 1.00 M ZnSO4 and placing a salt bridge to allow ion flow between the two solutions.a) What voltage will be produced by the cell and b) what metal is the anode? (Cd2+ + 2e- Cd; E° = -0.402 V)(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.8/5  (37)
(37)
Determine what causes the following electrolytic cell (which includes 50 g of metallic Ag and 1 L of 0.15 M Cu(NO3)2 ) to cease operation and determine how long the cell can sustain a current of 5 amps.
2 Ag (s) + Cu+2 (aq) 2 Ag+ (aq) + Cu (s)
(Short Answer)
4.8/5  (41)
(41)
If the coefficient of I- is 1, determine the number of electrons transferred:
OCl- + I- Cl- + IO-
(Short Answer)
4.9/5  (43)
(43)
Determine the coefficient for Sn+2 in the following balanced redox reaction.MnO4- + Sn2+ Sn4+ + Mn2+ (acidic solution)
(Multiple Choice)
4.8/5  (44)
(44)
The lead-acid battery used in automobiles utilizes the following redox reaction:
PbO2(s) + Pb (s) + 2 HSO4- (aq) + 2 H3O+ (aq) 2 PbSO4(s) + 4 H2O (l) E°= 2.04 VWhat mass of H2 being oxidized by O2 under standard acid conditions would be required to give the same amount of electrons as one mole of lead oxide?(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.7/5  (38)
(38)
Assign oxidation numbers to all the elements in sodium bicarbonate, NaHCO3.
(Short Answer)
4.7/5  (39)
(39)
Consider the redox reaction of triiodide and oxygen:I3- + O2 I2 + OH- (basic solution)If the coefficient of I3- is 4 in the balanced equation, what is the coefficient of OH-?
(Multiple Choice)
4.9/5  (32)
(32)
For the reaction given below, identify the anode and describe what happens to the electrode as the reaction continues.
3Fe(s) + Cr2O72-(aq) + 14 H+(aq) 3Fe2+(aq) + 2 Cr+3(aq) + 7H2O(l)
(Multiple Choice)
4.8/5  (32)
(32)
Which elements are changing oxidation states in the following reaction?
Zn(s) + 2 MnO2(s) + H2O(l) Zn(OH)2(s) + Mn2O3(s)
(Multiple Choice)
4.8/5  (39)
(39)
Calculate the standard free energy change for the redox reaction between silver ion and copper to give copper (II) and silver metal.(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.8/5  (36)
(36)
Consult a table of reduction potentials (Table 17-1 in the text) and determine which two metals are capable of reducing iron (II) to iron under standard conditions.
(Multiple Choice)
4.9/5  (28)
(28)
Showing 21 - 40 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)