Exam 17: Electron Transfer Reactions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
You have an abundant supply of NaCl salt from which you would like to prepare pure metallic sodium.
(Multiple Choice)
4.9/5  (35)
(35)
Calculate the standard free energy change for the following redox reaction: ( G = 77.11 (Ag+) and 65.49 kj/mol
(Cu+2)2 Ag+ (aq) + Cu (s) 2 Ag (s) + Cu2+ (aq)
(Short Answer)
4.9/5  (46)
(46)
At an engine block rebuilding factory you are in charge of replating Mn on the interiors of engine blocks. Based on the surface area and thickness needed, you determine that you need 35g of Mn to plate out by performing electrolysis on the engine block. Your plating solution is 3 M Mn(NO3)2. How long do you need to perform electrolysis if your machine performs at 220 Amps?
(Short Answer)
4.9/5  (36)
(36)
Which of the following combinations would provide the largest potential for a battery?(If needed, refer to Table 17-1in the text)
(Multiple Choice)
4.8/5  (34)
(34)
What is the correct description, in line notation, for an electrochemical cell comprised of only Ag wire, AgNO3 electrolyte solution, and a salt bridge having G = -2 kJ?If needed, use the following equation: G° = -nFE°, E° 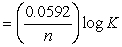 , E = E?
-
, E = E?
- 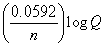 , moles e- =
, moles e- = 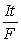 (If needed, refer to Table 17-1 in the text )
(If needed, refer to Table 17-1 in the text )
(Multiple Choice)
4.8/5  (37)
(37)
You determine that for proper protection of an engine part you need to put a coating of 3.0g of Cr(s) on your part. How long do you need to perform electrolysis on your engine part if your current is 30.0 Amps, and your Cr is in the form of Cr(NO3)3(aq)?
(Short Answer)
4.8/5  (43)
(43)
How is aluminium protected from oxidation?(If needed, refer to Table 17-1in the text)
(Multiple Choice)
4.7/5  (30)
(30)
Consider the redox reaction of permanganate and sulphur:
MnO4- + S Mn2+ + SO42- (acidic solution)If the coefficient of MnO4- is 6 in the balanced equation, what is the coefficient of H2O?
(Multiple Choice)
4.7/5  (32)
(32)
For the galvanic cell shown in the diagram, identify the anode and mark which direction the cations are moving in the salt bridge. 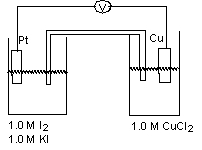
(Short Answer)
4.9/5  (33)
(33)
Draw three molecular pictures illustrating direct electron transfer in the reaction of silver (I) ions with copper metal.
(Essay)
4.8/5  (42)
(42)
For the working galvanic cell shown at standard conditions, how would you increase the cell potential?(If needed, refer to Table 17-1 in the text ) 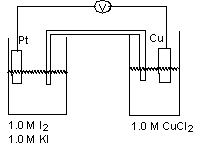
(Multiple Choice)
4.9/5  (37)
(37)
Use the half-reaction method to balance the following redox reaction:
I2 + S2O32- I - + S4O62- (basic solution)
(Short Answer)
4.8/5  (42)
(42)
Aluminium is used in a battery in which the following reaction occurs:
4 Al (s) + 3 O2 (g) + 4 OH- (aq) + 6 H2O 4 Al(OH) (-,4) (aq)If the battery must supply a current of 78 A for 4.0 hours, what mass of Al (ing) must be contained in the battery?(If needed, refer to Table 17-1 in the text)
(Short Answer)
4.7/5  (36)
(36)
Calculate the equilibrium constant for the following redox reaction:
Fe3+ (aq) + Cu+ (aq) Fe2+ (aq) + Cu2+ (aq)[E°(Fe3+, 2+) = 0.771 V] [E°(Cu2+,1+) = 0.153 V](If needed, refer to Table 17-1 in the text)
(Short Answer)
4.7/5  (31)
(31)
For the following galvanic cell what will be its potential when the reaction reaches equilibrium?(If needed, refer to Table 17-1in the text ) 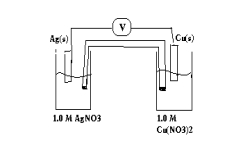
(Multiple Choice)
4.9/5  (45)
(45)
Showing 61 - 76 of 76
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)