Exam 16: Applications of Aqueous Equilibria
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Calculate the pH in the titration of a 0.325 g sample of acetylsalicylic acid (see line drawing below) initially in 25.0 mL water with 0.102 M NaOH when (a) 6.23 mL and (b) 20.00 mL of titrant have been added. 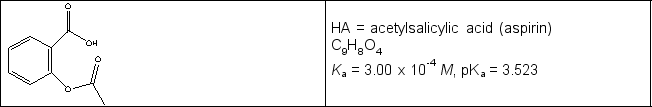
(Short Answer)
4.7/5  (39)
(39)
The pKa's of the three acid-conjugate base pairs derived from phosphoric acid, H3PO4, are 2.12, 7.21 and 12.32. How many g of sodium hydroxide would have be added to 150 mL of 0.1 M H3PO4 to prepare a buffer of pH = 7.41?
(Multiple Choice)
5.0/5  (41)
(41)
An aqueous solution is 0.2 M in both Mg2+ and Pb2+ ions. You wish to separate the two metal ions by adding oxalate, C2O42-. What is the concentration of lead when Mg2+ begins to precipitate?Ksp (MgC2O4) = 8.5 x 10-5 Ksp (PbC2O4) = 2.7 x 10-11
(Short Answer)
4.8/5  (41)
(41)
Consider the titration of 25.0 mL of an 0.11 M lactic acid solution CH3CH(OH)COOH (Ka= 8.4 x10-4) with 0.150 M NaOH.a) Calculate the pH after 10 mL of the NaOH solution has been added.b) Calculate the pH of the solution at 10 mL of NaOH past the stoichiometric point.c) Would phenol red (pKin = 7.9) or phenolphthalein (pKin = 9.4) be a better indicator for this titration?
(Short Answer)
4.8/5  (41)
(41)
What is the pH of the resulting solution when 0.1 moles of HCl are added to 1 L of solution containing 0.12 moles of aqueous Na2CO3?If needed, use the following equation: pH = pKa + log(A-/HA)
(Short Answer)
4.9/5  (37)
(37)
Trisbipyridylruthenium (II), Ru2+(BIPY)3, has been used in many studies, most recently to understand electrical conductivity in DNA molecules. Write all the equilibria in the formation of this complex.
(Essay)
4.8/5  (39)
(39)
What mass of sodium acetate must be added to 250 mL of 0.10 M acetic acid (pKa = 4.75) to give a buffer of pH = 4.90?
(Short Answer)
4.9/5  (35)
(35)
Write the equation showing how Na2HPO4 and NaH2PO4 act as a buffer. Also write an equation showing how this buffer reacts when HCl is added.
(Essay)
4.8/5  (34)
(34)
What is the concentration of phosphate in a solution made from a 0.15 M phosphoric acid whose pH has been adjusted to 7.6 by the addition of solid sodium hydroxide (assume no volume change)?
(Short Answer)
4.8/5  (26)
(26)
Your friend at Podunk U. urgently emails asking if you remember any acid-base chemistry. His problem is that for his final organic lab problem he needs to report the pKa of his acid. He did the pH titration, but only wrote down the following data for the titratant volumes: V = 14.27 mL; pH = 4.73. Vequivalence point = 22.43 mL. What is the pKa of the unknown acid?
(Multiple Choice)
4.9/5  (29)
(29)
Which of the following is NOT one of the equilibria in the complexation of Ru(II) by ammonia (coordination number = 6)?
(Multiple Choice)
4.9/5  (43)
(43)
Use the concepts of Ksp and the common-ion effect to calculate solution concentrations.
(Essay)
4.8/5  (39)
(39)
A buffer is made by adding 0.82 moles of monohydrogen phosphate and 0.82 moles of dihydrogen phosphate to sufficient water to give a total volume of 1.0 L. Hydrogen chloride, 0.60 moles, is absorbed into the solution. What is the pH?
(Short Answer)
4.8/5  (37)
(37)
What is the solubility product expression for barium phosphate?
(Multiple Choice)
4.7/5  (43)
(43)
Two buffer solutions of the same pH are prepared. After adding 15 millimoles of acid to 250 mL of one solution, the pH changes by 0.02 pH unit. Adding 15 millimoles of acid to 250 mL of the second solution results in a 0.05 pH unit change. Which statement most accurately describes the differences between the two buffer solutions?
(Multiple Choice)
4.9/5  (29)
(29)
A 1.32 g sample of solid CuCl2•2H2O is added to 1.21 L of 2.0 M aqueous ammonia. The formation constant for tetra-amminecopper (II) is 1.0 x 1012. What is the concentration of aqueous copper (II) in this solution?
(Short Answer)
4.7/5  (41)
(41)
What is the solubility product expression for iron (III) hydroxide?
(Multiple Choice)
4.8/5  (31)
(31)
Write the formation constant expression for the formation of [Zn(OH)4]-2 from Zn(OH)2(s).Zn(OH)2(s) + OH-(aq) ![Write the formation constant expression for the formation of [Zn(OH)<sub>4</sub>]<sup>-2</sup> from Zn(OH)<sub>2(s)</sub>.Zn(OH)<sub>2(s)</sub> + OH<sup>-</sup>(aq) [Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq)[Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq) + OH<sup>-</sup>(aq) [Zn(OH)<sub>4</sub>]<sup>-2</sup>(aq)](https://storage.examlex.com/TB9687/11ee726d_d438_fc12_827e_316527241e86_TB9687_11.jpg) [Zn(OH)3]-1(aq)[Zn(OH)3]-1(aq) + OH-(aq)
[Zn(OH)3]-1(aq)[Zn(OH)3]-1(aq) + OH-(aq) ![Write the formation constant expression for the formation of [Zn(OH)<sub>4</sub>]<sup>-2</sup> from Zn(OH)<sub>2(s)</sub>.Zn(OH)<sub>2(s)</sub> + OH<sup>-</sup>(aq) [Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq)[Zn(OH)<sub>3</sub>]<sup>-1</sup>(aq) + OH<sup>-</sup>(aq) [Zn(OH)<sub>4</sub>]<sup>-2</sup>(aq)](https://storage.examlex.com/TB9687/11ee726d_d438_fc13_827e_15dfae61f4d8_TB9687_11.jpg) [Zn(OH)4]-2(aq)
[Zn(OH)4]-2(aq)
(Essay)
4.9/5  (33)
(33)
How many grams of AgNO3 will dissolve in 1.32 L of a pH = 12.23 solution (Ksp AgOH = 1.52 x 10-8)?
(Short Answer)
4.8/5  (33)
(33)
Showing 41 - 60 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)