Exam 16: Applications of Aqueous Equilibria
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
What is the pH upon adding 10 mL of 1.0 M NaOH toa) 1.2 L of water and b) a solution made by the addition of 0.34 mole of Na2HPO4 and 0.65 mole of NaH2PO4 and sufficient water to give a total volume of 1.2 L?If needed, use the following equation: pH = pKa + log(A-/HA)
(Short Answer)
4.9/5  (36)
(36)
Calculate the pH in the titration of a 0.325 g sample of acetylsalicylic acid (see line drawing below) initially in 25.0 mL water with 0.102 M NaOH when (a) 8.84 mL and (b) 17.68 mL of titrant have been added. 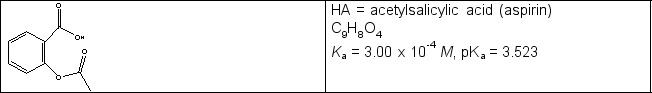
(Short Answer)
4.7/5  (36)
(36)
A solution is made by the addition of 0.34 mole of Na2HPO4 and 0.65 mole of NaH2PO4 and sufficient water to give a total volume of 1.2 L. How many grams of NaOH would need to be added to increase the pH by 0.2 pH units?If needed, use the following equation: pH = pKa + log(A-/HA)
(Multiple Choice)
4.7/5  (32)
(32)
At what pH would a solution with a total concentration of 0.20 M phosphate NOT be a buffer? Explain your answer.
(Short Answer)
4.8/5  (33)
(33)
1.1 mg of HgS (Ksp1.6 x 10-54) is added to 1.0 L of 0.01 M NaCN ((Hg(CN)4+2), Kf = 4 x 1041)? What is the value of the reaction quotient for Qsp and will all of the HgS dissolve?
(Essay)
4.9/5  (27)
(27)
What mass of ammonium chloride (pKa (NH4+) = 9.25) must be added to 2.0 L of 0.65 M aqueous ammonia to give a buffer with pH = 8.75?
(Short Answer)
4.9/5  (35)
(35)
Showing 61 - 66 of 66
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)