Exam 9: Properties of Solutions
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
The interactions that play an important role in the dissolution of a salt in water are
I. ion-dipole
II. hydrogen bonding
III. dipole-dipole
IV. ion-ion
(Multiple Choice)
4.7/5  (40)
(40)
Classify the following molecules or fragments as hydrophobic or hydrophilic. A line from an atom indicates where a bond would be formed to another fragment.
1. the -CH2SO3- group
2. CH3CH2OH
3 the -CH2(CH2)11CH3 group
4. the -NH3+ group
(Multiple Choice)
4.9/5  (31)
(31)
What is the molarity of an aqueous solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
(Multiple Choice)
4.9/5  (41)
(41)
What is the molarity of a solution made from mixing 34 grams of CaCl2 with enough water to make 150 ml of solution?
(Multiple Choice)
4.7/5  (39)
(39)
Pesticides are non-polar molecules in general. What needs to be done so that farmers are able to spray aqueous solutions containing pesticides?
(Multiple Choice)
5.0/5  (41)
(41)
Consider a can containing 355 mL of a carbonated soft drink at 0°C under 2.2 atm CO2 pressure. What is the concentration of CO2 in the soda, assuming that KH = 7.8 x 10-2 M/atm?
(Short Answer)
4.8/5  (39)
(39)
The vapour pressure of toluene at 25oC is 3.79 KPa and that of benzene is 12.7 kPa. The vapour above a solution that has mole fraction of benzene = 0.5,
(Multiple Choice)
4.9/5  (27)
(27)
Which of the following are true solutions: milk, brass, NaCl in water, sugar in water, ink?
(Multiple Choice)
4.9/5  (40)
(40)
Which of the following has the substances arranged in order of increasing heat of fusion?
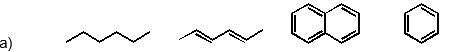

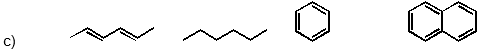

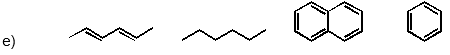
(Short Answer)
4.8/5  (38)
(38)
A solution is made from dissolving 102 grams of potassium carbonate in 100 grams of water. What is the solvent in this solute in the solution and explain.
(Essay)
4.8/5  (41)
(41)
Lysozyme is an enzyme found in tears whose function is to break down bacterial cell walls thus killing the bacteria and protecting the eye from infection. A solution made which contains 0.100 g of lysozyme in 150 g of water ( = 1.00 g/mL) at 25°C has an osmotic pressure of 8.9 Torr. What is the molar mass of this enzyme?
(Short Answer)
4.7/5  (34)
(34)
Understand some aspects of colloidal suspensions and surfactant solutions.
(Essay)
4.8/5  (42)
(42)
The greatest gas solubility for a gas in solution is predicted under what conditions?
(Multiple Choice)
4.8/5  (28)
(28)
What is the concentration of all ions in a solution that is 0.569 m CaCl2 (solution density 1.05 g/ml)?
(Multiple Choice)
4.7/5  (26)
(26)
What is the molality of a solution made from mixing 39 grams of sugar (MM = 180 g/mol) with 310 grams of water?
(Multiple Choice)
4.8/5  (39)
(39)
Predict the relative solubilities of a solute in various solvents, and explain in terms of intermolecular forces.
(Essay)
4.9/5  (40)
(40)
The molecule drawn below is Vitamin B1, thiamine. Would you predict this molecule to be more soluble in water or body fat and explain why? 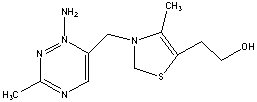
(Essay)
4.8/5  (37)
(37)
Which aqueous solution would be expected to have the highest boiling point?
(Multiple Choice)
4.8/5  (31)
(31)
Draw a molecular picture of a surfactant on a waxed surface, like soapy water on a freshly waxed car.
(Essay)
4.7/5  (35)
(35)
Showing 41 - 59 of 59
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)