Exam 2: The Behaviour of Gases
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
At 1000°C and 10.0 Torr, the density of a certain element in the gas phase is 2.9 x 10-3 g/L. What is the element?
(Multiple Choice)
4.9/5  (39)
(39)
The level of mercury on the sample side of a manometer is 156 mm below that of the atmospheric side. If the atmospheric pressure is 758 Torr, what is the pressure of the gas sample (in bar)?
(Short Answer)
4.9/5  (31)
(31)
A tank holds a mixture of nitrogen and chlorine gases. If the partial pressure of nitrogen is 3.3 atm and the total pressure of the mixture is 22,000 kPa; what is the mole fraction of chlorine gas?
(Short Answer)
4.9/5  (29)
(29)
A tank is pressurized with 5.0 atm of N2 and 10.0 atm of H2. Ammonia (NH3) is formed. When the pressure finally remains constant, indicating that the reaction has proceeded as far as it will go, the partial pressure of ammonia is 3.2 atm. What is the total pressure in the tank assuming that neither the temperature nor the volume of the container have changed?
(Short Answer)
4.9/5  (37)
(37)
If the density of a sample of nitrogen gas was 1.2 g/L at 298K; what is the density of chlorine gas at 298K if the pressure of the chlorine gas is twice that of the nitrogen?
(Short Answer)
4.9/5  (38)
(38)
A diver exhales a bubble with a volume of 250 ml at a pressure of 2.4 atm and 15˚C. What will be the volume of that bubble when it reaches the surface where the pressure if 1 atm and the temperature if 29˚C?
(Short Answer)
4.8/5  (35)
(35)
The following set up is arranged in a laboratory. Assuming the volume of the connecting tubing is negligible and there is no temperature change, determine the final total pressure if all three flasks were allowed to mix. 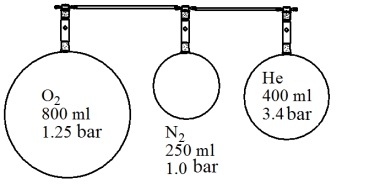
(Multiple Choice)
4.8/5  (39)
(39)
A sample of gas is cooled from 273°C to 0°C. Which of the following statements accurately describes the sample at its new conditions?
1) The kinetic energy is now nearly zero.
2) The average velocity is now twice as fast.
3) The average velocity is half as fast.
4) The average kinetic energy is halved.
5) The average velocity is about two thirds of the original.
(Multiple Choice)
4.7/5  (40)
(40)
A sample of gas is heated from 200 K to 400 K. Which of the following statements accurately describes the sample at its new conditions?
1) The average velocity is twice as fast.
2) The molecules have four times the kinetic energy.
3) The average velocity is nearly the same.
4) The average kinetic energy is half the original.
5) The kinetic energy is twice the original.
(Multiple Choice)
4.7/5  (40)
(40)
A cylinder of carbon dioxide is shipped to a research facility. This cylinder is under a pressure of 145 atm and has a volume of 95 litres. What volume would this same amount of gas fill if the valve was opened to the room, which is under a pressure of 745 mm Hg?
(Short Answer)
4.7/5  (45)
(45)
If the rate of effusion of Kr is 5 x 10-5 moles/s, what is the rate of effusion of chlorine gas under identical conditions in units of molecules/s?
(Short Answer)
5.0/5  (37)
(37)
If the density of a sample of nitrogen gas was 8.0 x 10-3 g/L, what is the density of He under identical conditions of temperature and pressure?
(Short Answer)
4.7/5  (37)
(37)
One group of compounds that contain only carbon and hydrogen are called the cycloalkanes and have the empirical formula CH2. The vapour from a sample of one of these compounds occupies a volume of 253.2 mL at a temperature of 99.8°C and a pressure of 754.8 Torr. The vapour has a mass of 0.5921g. What is the molecular formula of the compound?
(Short Answer)
4.8/5  (34)
(34)
At an elevation of 10 km above sea level (about 33,000 feet, approximately the cruising altitude of a jet airliner), the atmospheric pressure is 210 Torr and the temperature is 230K. Assuming air is 21% O2, how many L of air at this altitude would be required to completely burn 1 kg of jet fuel, C12H26?
(Short Answer)
4.8/5  (36)
(36)
A sample of N2 gas is contaminated with a gas. It is found that that contaminant effuses at 0.36 times the rate of N2. What is the contaminating gas?
(Multiple Choice)
4.9/5  (32)
(32)
A sample of oxygen is expected to diffuse ______ than chlorine gas.
(Multiple Choice)
4.7/5  (41)
(41)
A steel cylinder 1.2 m tall and 20 cm in diameter is filled with nitrogen at a pressure of 152 bar at a temperature of 24°C. How many kg of nitrogen does the cylinder contain?
(Short Answer)
4.9/5  (31)
(31)
In the summer there is frequently dew on the grass on humid nights. What will the dew point be if during the afternoon there was a relative humidity of 78% at 32˚C (90˚F)? 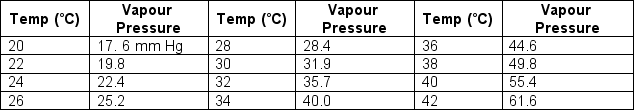
(Short Answer)
4.9/5  (35)
(35)
Professional cyclists can metabolize more than 80 mL of oxygen per kg of body weight per minute (sometimes called the V-O2 max). Calculate the mass of glucose (in grams) that could theoretically be consumed by the following reaction by a 70 kg cyclist in an hour (assume 25oC and 1 atm pressure).
C6H12O6 + 6 O2 6 CO2 + 6 H2O
(Multiple Choice)
4.8/5  (34)
(34)
Showing 41 - 60 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)