Exam 2: The Behaviour of Gases
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Use stoichiometry to solve problems involving gas-phase chemical reactions.
(Short Answer)
4.8/5  (32)
(32)
Increasing the number of molecules in a container increases the pressure because
(Multiple Choice)
4.9/5  (38)
(38)
A reaction is carried out under an atmosphere of oxygen. As the reaction proceeds, oxygen is consumed. The reaction started in a 500 ml flask, under a pressure of 1015 mm Hg, and a temperature of C. How many moles of oxygen had been consumed if at the end of the reaction the pressure was 790 mm Hg and the temperature did not change?
(Multiple Choice)
4.9/5  (29)
(29)
Athletes are reminded to drink more water when they are exercising in cold weather because it is easy to get dehydrated. The main reason for this is
(Multiple Choice)
4.8/5  (31)
(31)
Explain the basic concepts of kinetic molecular theory: molecular speed, energy, and the effects of temperature and volume on gas pressure.
(Essay)
4.8/5  (38)
(38)
A gas mixture containing oxygen, nitrogen, and helium exerts a total pressure of 925 Torr. If the partial pressures are oxygen 425 Torr, and helium 75 Torr, what is the partial pressure (in bar) of nitrogen in the mixture?
(Short Answer)
4.8/5  (38)
(38)
Carbon monoxide is used frequently in the production of metals and steel. A cylinder of CO is shipped to a research facility with a pressure of 170. atm and a volume of 90.0 litres. How many kilograms of CO are needed to fill the cylinder at 10.0˚C?
(Short Answer)
4.8/5  (39)
(39)
A 1.56 L sample of nitrogen gas (1.21 x 10-2g) is heated from 24°C to 350°C. Calculate the densities of the nitrogen at the different temperatures.
(Essay)
4.8/5  (40)
(40)
Which of the following molecules/atoms will exert a greater force when it strikes the wall of the container under ideal conditions?
(Multiple Choice)
4.8/5  (36)
(36)
A 400 L steel tank is filled with a mixture of 20 kg of methane (CH4), 5 kg of ethane (C2H6) and 0.050 g of butanethiol (C4H10S). (This last sulphur-containing compound has an intense smell to indicate any leaks of this flammable mixture). What is the total pressure in the tank and the partial pressure of each component if the temperature is 24 °C?
(Short Answer)
4.9/5  (37)
(37)
Methanol, CH3OH, is produced by the reaction of methane with oxygen under carefully controlled conditions:
2 CH4 (g) + O2 (g) 2 CH3OH (g)
A 200 L reactor is filled with a mixture of methane to a pressure of 10.5 atm and 3.50 atm oxygen at 298 K. The reactor is then heated to 350°C.(a) What is the mass of methanol formed if the reaction proceeds to 100% yield?(b) What are the partial pressures of each of the substances and the total pressure in the reactor at the end of the reaction if the temperature is 350°C?
(Essay)
4.7/5  (36)
(36)
An ozone measurement in a ultraviolet paint curing facility is 12.2 PPM. The air pressure is 752.1 mm Hg. What is the partial pressure of ozone in the facility?
(Multiple Choice)
4.8/5  (34)
(34)
Calculate gas densities and molar masses from pressure-volume-temperature data.
(Essay)
4.8/5  (42)
(42)
A tank holds a mixture of oxygen and nitrogen gases. If the partial pressure of oxygen is 3.3 atm and the mole fraction of N2 gas X(N2) = 0.78, what are the total pressure in the tank and the partial pressure of N2?
(Multiple Choice)
4.9/5  (37)
(37)
A sample of radon is expected to diffuse ______ than oxygen gas.
(Multiple Choice)
4.9/5  (42)
(42)
On the graph provided draw the speed distribution of O2 at 250K, O2 at 350 K, and H2 at 250K. 
(Essay)
4.8/5  (38)
(38)
Acetic acid, shown below, is the substance responsible for the odour of vinegar. 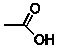 What is the average velocity and average kinetic energy (kJ/mol) of acetic acid molecules?
What is the average velocity and average kinetic energy (kJ/mol) of acetic acid molecules?
(Short Answer)
4.7/5  (40)
(40)
A tank holds a mixture of argon and helium gases. If the partial pressure of oxygen is 3.3 atm and the total pressure of the mixture is 15 atm; what is the mole fraction of helium gas?
(Multiple Choice)
4.9/5  (38)
(38)
If a 0.18 g sample of aluminum metal is dropped into 400 mL of 6 M HCl, hydrogen gas is evolved. The hydrogen evolved is collected over water, so the gas collected is a mixture of water vapour (vapour pressure = 23.8 mm Hg) and hydrogen gas. If the external pressure is 1.02 atm and temperature is 25?C, what will be the total volume of gas collected in L?
(Multiple Choice)
4.8/5  (38)
(38)
Showing 61 - 80 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)