Exam 2: The Behaviour of Gases
Exam 1: Fundamental Concepts of Chemistry118 Questions
Exam 2: The Behaviour of Gases84 Questions
Exam 3: Energy and Its Conservation65 Questions
Exam 4: Atoms and Light81 Questions
Exam 5: Atomic Energies and Periodicity71 Questions
Exam 6: Fundamentals of Chemical Bonding71 Questions
Exam 7: Theories of Chemical Bonding78 Questions
Exam 8: Effects of Intermolecular Forces71 Questions
Exam 9: Properties of Solutions59 Questions
Exam 10: Organic Chemistrystructure57 Questions
Exam 11: Organic Chemistryreactions40 Questions
Exam 12: Spontaneity of Chemical Processes70 Questions
Exam 13: Kinetics: Mechanisms and Rates of Reactions77 Questions
Exam 14: Principles of Chemical Equilibrium70 Questions
Exam 15: Aqueous Acidbase Equilibria79 Questions
Exam 16: Applications of Aqueous Equilibria66 Questions
Exam 17: Electron Transfer Reactions76 Questions
Exam 18: Macromolecules83 Questions
Exam 19: The Transition Metals38 Questions
Exam 20: The Main Group Elements29 Questions
Exam 21: Nuclear Chemistry and Radiochemistry44 Questions
Select questions type
Vinyl chloride is a important industrial chemical used in many different applications. However, it has health impacts even at the PPB level. If an average breath is about 1.5 L, how many vinyl chloride molecules would be contained in one breath, assuming 1 atm pressure and 26°C if the vinyl chloride concentration is 10 PPB?
(Short Answer)
4.7/5  (36)
(36)
Which of the following molecules/atoms will exert a greater force when it strikes the wall of the container under ideal conditions?
(Multiple Choice)
4.8/5  (31)
(31)
Data for a pulsed molecular beam experiment at a temperature of 297 K is shown below: 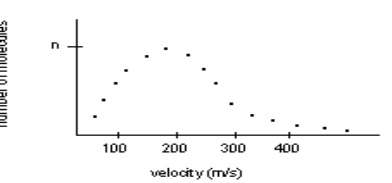 Which are the following are probably true about the sample?1. The sample consists of two different molar mass molecules.2. The sample has a lower molar mass than ammonia (avg is 490 m/s).3. The sample has a higher molar mass than ammonia (avg is 490 m/s).4. The sample consists of molecules with the same molar mass.5. The average velocity is near 200 m/s.
Which are the following are probably true about the sample?1. The sample consists of two different molar mass molecules.2. The sample has a lower molar mass than ammonia (avg is 490 m/s).3. The sample has a higher molar mass than ammonia (avg is 490 m/s).4. The sample consists of molecules with the same molar mass.5. The average velocity is near 200 m/s.
(Multiple Choice)
4.8/5  (35)
(35)
Calculate the pressure of a gas under non-ideal conditions, and explain the deviations from ideality.
(Essay)
4.7/5  (32)
(32)
Showing 81 - 84 of 84
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)