Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Use the following information to answer
H2Se: Ka1 = 1.0 x 10-4, Ka2 = 1.0 x 10-10
-If sodium selenide is added to pure water, we would expect the pH to:
(Multiple Choice)
4.8/5  (42)
(42)
What is the pH of a solution that is 1.7 x 10-4 M in H3O+?
(Multiple Choice)
4.9/5  (35)
(35)
Methanol is described by the following skeleton structure.
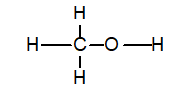 Which substance is formed when CH3OH acts as a Brønsted base?
Which substance is formed when CH3OH acts as a Brønsted base?
(Multiple Choice)
4.9/5  (29)
(29)
Hydrogen peroxide has been used as a bleach to change hair color, as a disinfectant to treat wounds and as a rocket fuel. It is also a weak acid. Calculate the pH of a 0.018 M H2O2 solution. (H2O2: Ka = 2.2 x 10-12)
(Multiple Choice)
4.8/5  (35)
(35)
Showing 121 - 125 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)