Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which of the following statements is true for a 1.0 M solution of the strong acid HCl in water?
(Multiple Choice)
4.8/5  (40)
(40)
Rank the following 0.100 M solutions in order of increasing pH (from lowest pH to the highest).
HNO3, KI, HIO3, HIO4
(Multiple Choice)
4.8/5  (20)
(20)
Which of the following solutions would have the lowest pH?
(HCO2H: Ka = 1.8 x 10-4; NH4+: Ka = 5.6 x 10-10)
(Multiple Choice)
4.9/5  (45)
(45)
Which of the following solutions will show the largest change in pH when
10 mL of 1 M NaOH is added to it?
(Multiple Choice)
4.9/5  (30)
(30)
Sodium fluoride, NaF (a soluble salt), is dissolved in water. Which one of the following correctly describes the relationships between the concentrations of the species present at equilibrium?
(Multiple Choice)
4.9/5  (36)
(36)
What is the value of Kb for the formate (HCO2-) ion if Ka for formic acid (HCO2H) is 1.8x10-4?
(Multiple Choice)
4.9/5  (40)
(40)
Kb for methylamine (CH3NH2) is the equilibrium constant for which reaction?
(Multiple Choice)
4.8/5  (40)
(40)
Of the following, which is the strongest acid (lone pairs omitted for clarity)?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following compounds would give a 0.10 M solution with a pH less than 7?
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following structural factors is primarily responsible for H2Se being a stronger acid than H2S?
(Multiple Choice)
4.9/5  (42)
(42)
What is the pOH of a 0.025M solution of hydrobromic acid (Ka = 1 x 109)?
(Multiple Choice)
4.8/5  (37)
(37)
There are many ways of "fluoridating" water. One approach involves adding a salt of the fluoride ion, such as NaF. Calculate the pH of a 0.15 M NaF solution. (HF: Ka = 7.2 x 10-4)
(Multiple Choice)
5.0/5  (34)
(34)
The compound HB can be represented as 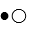 where
where 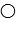 represent B- and
represent B- and  represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.
represents H+. Given this, ___ would represent the conjugate acid of HB and ____ would represent the conjugate base.
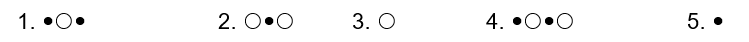
(Multiple Choice)
4.9/5  (39)
(39)
Rank the following in order of increasing pH of 0.1M solutions.
NaI, HI, HIO3, NaIO3
(Essay)
4.8/5  (35)
(35)
Predict the products of the following acid-base reaction.
HOCl(aq) + CH3NH2(aq) 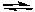
(Essay)
4.7/5  (38)
(38)
Using a table of Ka's, determine which of the following is the strongest Brønsted acid.
(Multiple Choice)
4.9/5  (37)
(37)
Showing 101 - 120 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)