Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
The dissociation of water into H3O+ and OH- is an endothermic process. As the temperature of pure water is raised, the degree to which water dissociates ________ and the pH ________.
(Multiple Choice)
4.7/5  (45)
(45)
Which of the following acid-base reactions should go more or less to completion?
(Multiple Choice)
4.8/5  (37)
(37)
Sulfurous acid (H2SO3) is an acid and the sulfite ion (SO32-) is a base. Which equation correctly describes the relationship between the equilibrium constants for these compounds?
(Multiple Choice)
4.8/5  (39)
(39)
When NH4Cl is dissolved in water the following species will be present: NH4+, Cl - OH - , H3O+, and NH3. Which of the following represents the correct relationship between the species in solution?
(Multiple Choice)
4.9/5  (45)
(45)
A solution is known to have a hydronium ion concentration of 1.0 x 10-6 M. What percentage of the total H3O+ ion concentration comes from the dissociation of water?
(Multiple Choice)
4.8/5  (33)
(33)
Which of the following represents a conjugate acid/base pair?
(Multiple Choice)
4.8/5  (35)
(35)
NaHCO3 can be used to neutralize strong bases, such as NaOH. What conclusion can be drawn from the fact that the following acid-base reaction proceeds to the right as written?
HCO3-(aq) + OH-(aq) 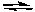 CO32-(aq) + H2O(l)
CO32-(aq) + H2O(l)
(Multiple Choice)
4.9/5  (44)
(44)
Which equation best describes a 0.10 M solution of a strong acid such as hydroiodic acid (HI: Ka = 3 x 109)?
(Multiple Choice)
4.7/5  (47)
(47)
Which of the following compounds aren't acids in water?
(I) NH4Cl (II) HNO3 (III) NH3 (IV) HI (V) K2S
(Multiple Choice)
4.7/5  (38)
(38)
Use the relationship between Ka1 and Ka2 for H2SO3 and Kb1 and Kb2 for the SO32- ion to predict whether a 0.10 M solution of the HSO3- (bisulfite) ion would be acidic, basic or neutral. (H2SO3: Ka1 = 1.7 x 10-2, Ka2 = 6.4 x 10-8)
(Multiple Choice)
4.8/5  (37)
(37)
For a particular weak acid, HA, in pure water, as the initial concentration of the weak acid increases, the acid-dissociation equilibrium constant of that acid should
(Multiple Choice)
5.0/5  (33)
(33)
Which of the following equations can be used to calculate Kb1 and Kb2 for Na2Gly from Ka1 and Ka2 for H2Gly?
(Multiple Choice)
5.0/5  (42)
(42)
When 1.0 x 10-5 mole of HOCl (Ka = 3.5 x 10-8) is dissolved in pure water and diluted to 1.00 L, which assumption can't be applied in the calculation of the pH of this solution?
(Multiple Choice)
4.7/5  (40)
(40)
Predict the products of the following acid-base reaction.
NaOCH3(aq) + NaHCO3(aq) 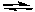
(Essay)
4.8/5  (37)
(37)
Which of the following solutions has a pH greater than 7.0?
(Multiple Choice)
4.7/5  (38)
(38)
What can we conclude from the fact that the following reaction proceeds as written?
NaNH2(s) + H2O(l) 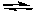 NH3(aq) + OH-(aq)
NH3(aq) + OH-(aq)
(Multiple Choice)
4.8/5  (34)
(34)
Showing 41 - 60 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)