Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which of the following solutions would have a pH greater than 7.0?
(Multiple Choice)
4.9/5  (32)
(32)
The hydride ion, (H-), is a stronger base than the hydroxide ion, (OH-). The products of the reaction between H- (aq) and H2O(l) would be.
(Multiple Choice)
4.7/5  (33)
(33)
Consider the following reaction:
H2PO4-(aq) + HCO3-(aq) 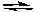 H2CO3(aq) + HPO42-(aq)
Brønsted would identify the acidic species as:
H2CO3(aq) + HPO42-(aq)
Brønsted would identify the acidic species as:
(Multiple Choice)
4.9/5  (36)
(36)
What would happen if more formic acid (HCO2H) was added to the following solution at equilibrium?
HCO2H(aq) + H2O(l) 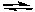 H3O+(aq) + HCO2-(aq)
H3O+(aq) + HCO2-(aq)
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following groups contains salts that all form basic solutions in water?
(Multiple Choice)
4.8/5  (39)
(39)
A solution of weak base is added to a beaker of water. Which of the following will be true as the base is added to the water?
(Multiple Choice)
4.9/5  (44)
(44)
Which of the following is correct (assume all solutions have the same concentration)?
(Multiple Choice)
4.9/5  (26)
(26)
Use the following information to answer
H2Se: Ka1 = 1.0 x 10-4, Ka2 = 1.0 x 10-10
-The base-ionization constant for the Se2- ion would be:
(Multiple Choice)
4.8/5  (35)
(35)
What reaction would take place to limit the change in pH when NaOH is added to a mixture of acetic acid (CH3CO2H) and acetate ion (CH3CO2-)?
(Multiple Choice)
4.9/5  (42)
(42)
The addition of sodium formate (HCO2Na) to a solution containing formic acid (HCO2H: Ka = 1.8 x 10-4) will cause
(Multiple Choice)
4.9/5  (31)
(31)
Calculate the pH of a solution prepared by dissolving 1.00 x 10-2 moles of sodium hypochlorite (NaOCl) in enough water to produce a 1.00 L of solution. Hypochlorous acid is a weak monoprotic acid with Ka = 3.2 x 10-8.
(Multiple Choice)
4.9/5  (38)
(38)
Calculate the pH of a buffer prepared by mixing 0.10 mol of sodium formate and 0.05 mol of formic acid in 1.0 L of solution. (HCO2H: Ka = 1.8 x 10-4)
(Multiple Choice)
4.7/5  (40)
(40)
At what point in the following titration curve would the pH of the solution be equal to the pKa of the acid?
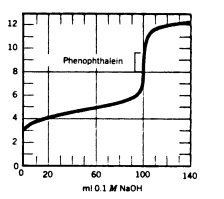
(Multiple Choice)
4.7/5  (41)
(41)
Calculate the approximate pH of a 0.100 M solution of formic acid (HCO2H) in water. (HCO2H: Ka = 1.8 x 10-4)
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following equations is valid for a 0.10 M solution of formic acid, HCO2H?
HCO2H(aq) + H2O(l) 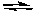 HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4
HCO2-(aq) + H3O+(aq) Ka = 1.8 x 10-4
(Multiple Choice)
4.7/5  (35)
(35)
What is the H3O+ ion concentration in a 0.10 M NH3 solution?
(Kb = 1.8 x 10-5)
(Multiple Choice)
4.8/5  (36)
(36)
Ammonia and the ammonium ion form a conjugate acid-base pair.
NH3(aq) + H2O(l) 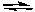 NH4+(aq) + OH-(aq)
Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10.
NH4+(aq) + OH-(aq)
Calculate the pH of 0.10 M NH3 if Ka for the NH4+ ion is 5.6 x 10-10.
(Multiple Choice)
4.8/5  (33)
(33)
Use the following acid-dissociation equilibrium constants for
 -A solution is prepared with initial concentrations of HA and NaA of 0.50 M and 1.00 M respectively. What is the pH of the solution?
-A solution is prepared with initial concentrations of HA and NaA of 0.50 M and 1.00 M respectively. What is the pH of the solution?
(Multiple Choice)
4.9/5  (32)
(32)
Showing 21 - 40 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)