Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
What is the conjugate acid of hydrogen carbonate ion, HCO3 - ?
(Multiple Choice)
4.7/5  (37)
(37)
When would the pH of a solution prepared by adding sodium formate to formic acid be equal to the pKa of formic acid, HCO2H?
(Multiple Choice)
5.0/5  (42)
(42)
What is the pH of a solution that is 0.01 M in CH3NH2 and 0.1 M in CH3NH3+?
CH3NH2(aq) + H2O(l) 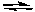 CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
CH3NH3+(aq) + OH-(aq) Kb = 4.4 x 10-4
(Multiple Choice)
4.9/5  (41)
(41)
Which of the following would be the strongest Brønsted base?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following is the best Lewis structure for carbonic acid (H2CO3)?
(Multiple Choice)
4.9/5  (37)
(37)
A diprotic acid, H2A, has the following dissociation constants:
Ka1 = 2.1 x 10-7 and Ka2 = 4.3 x 10-13. The H3O+ ion concentration when this acid is dissolved in water is
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following acids would have the strongest conjugate base?
(Multiple Choice)
4.9/5  (46)
(46)
The salt NaF is dissolved in water. Which of the following relationships would be true for the species in solution?
(Multiple Choice)
5.0/5  (39)
(39)
Explain the following observation: Water becomes acidic when CO2 is bubbled into it.
(Essay)
4.7/5  (42)
(42)
For a weak diprotic acid, H2A, for which Ka1 = 2.1 x 10-7 and
Ka2 = 4.3 x 10-13 the A2- ion concentration at equilibrium will be:
(Multiple Choice)
4.9/5  (30)
(30)
Which of the following compounds can act as either a Brønsted acid or a Brønsted base?
(I) NaHCO3 (II) Na2CO3 (III) H2CO3 (IV) CO2 (V) H2O
(Multiple Choice)
4.8/5  (46)
(46)
Which of the following would you expect to be the strongest acid?
(Multiple Choice)
4.8/5  (36)
(36)
What is the hydroxide ion concentration in a pH = 5.14 solution?
(Multiple Choice)
4.8/5  (37)
(37)
What is the pH of a 0.10 M H2CO3 solution?
(H2CO3: Ka1 = 4.2 x 10-7, Ka2 = 4.8 x 10-11)
(Multiple Choice)
5.0/5  (38)
(38)
Ethanol (CH3CH2OH), like water, can behave both as an acid or as a base. Which of the following structures corresponds to the Brønsted conjugate acid and conjugate base of ethanol?

(Multiple Choice)
4.8/5  (42)
(42)
Calculate the approximate pH of a 0.10 M lactic acid solution.
(Ka = 8.4 x 10-4)
(Multiple Choice)
4.8/5  (35)
(35)
Determine the [OH ] for an aqueous solution at 25 ![Determine the [OH <sup></sup> ] for an aqueous solution at 25 C with a pH of 4.80.](https://storage.examlex.com/TB9692/11ee9f01_38f8_89d9_9fac_1f2262b4b888_TB9692_11.jpg) C with a pH of 4.80.
C with a pH of 4.80.
(Multiple Choice)
4.8/5  (32)
(32)
Use the following acid-dissociation equilibrium constants for
 -0.5 M solutions of NaA, NaB, NaC, and NaD are prepared. Which solution is the most basic?
-0.5 M solutions of NaA, NaB, NaC, and NaD are prepared. Which solution is the most basic?
(Multiple Choice)
4.7/5  (34)
(34)
Which of the following structural factors is primarily responsible for H2AsO4 - being a stronger acid than HAsO42 - ?
(Multiple Choice)
4.9/5  (38)
(38)
Arrange the following bases in order of increasing strength: NH3, PH3, H2O and H2S.
(Essay)
4.9/5  (34)
(34)
Showing 61 - 80 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)