Exam 11: Acids and Bases
Exam 1: Elements Compounds39 Questions
Exam 2: The Mole126 Questions
Exam 3: Structure of the Atom106 Questions
Exam 4: The Covalent Bond105 Questions
Exam 5: Ionic and Metallic Bonds80 Questions
Exam 6: Gases59 Questions
Exam 7: Making and Breaking of Bonds69 Questions
Exam 8: Liquids and Solutions54 Questions
Exam 9: Solids31 Questions
Exam 10: An Introduction to Kinetics and Equilibrium94 Questions
Exam 11: Acids and Bases125 Questions
Exam 12: Oxidation-Reduction Reactions81 Questions
Exam 13: Chemical Thermodynamics56 Questions
Exam 14: Kinetics79 Questions
Exam 15: Nuclear Chemistry41 Questions
Exam 16: Organic Chemistry30 Questions
Select questions type
Which one of the following mixtures would make the best buffer?
(Multiple Choice)
4.9/5  (34)
(34)
Succinic acid (HO2CCH2CH2CO2H) is an intermediate in the Kreb's cycle used to "burn" carbohydrates, proteins and lipids. The formula for succinic acid can be abbreviated as H2Sc.
H2Sc(aq) + H2O(l) 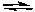 H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l)
H3O+(aq) + HSc-(aq) Ka1 = 6.9 x 10-5
HSc-(aq) + H2O(l) 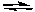 H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
Which of the following assumptions may not be valid for solutions of succinic acid because the difference between Ka1 and Ka2 for H2Sc is very small?
H3O+(aq) + Sc2-(aq) Ka2 = 2.5 x 10-6
Which of the following assumptions may not be valid for solutions of succinic acid because the difference between Ka1 and Ka2 for H2Sc is very small?
(Multiple Choice)
4.8/5  (32)
(32)
Use the following information to answer
H2Se: Ka1 = 1.0 x 10-4, Ka2 = 1.0 x 10-10
-In a 0.10 M solution of H2Se, the Se2- concentration is:
(Multiple Choice)
4.9/5  (38)
(38)
What is the pH of a solution that is simultaneously 0.10 M in both NH3 and the NH4+ ion?
(NH4+: Ka = 5.6 x 10-10)
NH3(aq) + H2O(l) 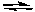 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
(Multiple Choice)
4.8/5  (42)
(42)
Which of the following won't produce an acidic solution when dissolved in water?
(Multiple Choice)
4.9/5  (32)
(32)
For H2CO3, Ka2 = 4.7 x 10-11. Which of the following numbers is closest to the pH of a 0.200 M Na2CO3 solution? (Hint: Only the first step in the hydrolysis of the CO32- ion need be considered in this calculation.)
(Multiple Choice)
4.9/5  (36)
(36)
The base-ionization constants for three bases, A(OH), B(OH), and C(OH), are 1.8 x 10-5, 4.4 x 10-4, and 7.4 x 10-4, respectively. Solutions of the bases having equal molarity have pH values that decrease in the following order:
(Multiple Choice)
4.8/5  (40)
(40)
HA is a weak monoprotic acid with Ka = 2.4 x 10-6. What is [OH-] in a 0.300 M solution of the salt, NaA?
(Multiple Choice)
4.8/5  (46)
(46)
Predict the products of the following acid-base reaction.
NH4Cl(aq) + NaSH(aq) 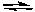
(Essay)
4.9/5  (33)
(33)
Assume a solution is prepared by adding 1.0 x 10-3 moles of a weak monoprotic acid (Ka = 2.0 x 10-4) to enough water to give a liter of solution. If you want to compute the H3O+ ion concentration with an error of 5% or less, you can legitimately ignore:
(Multiple Choice)
4.7/5  (30)
(30)
If equimolar amounts of NH3 and NH4Cl are dissolved in 1.00 L of water, what is the H3O+ ion concentration in this solution? (NH3: Kb = 1.8 x 10-5)
(Multiple Choice)
4.8/5  (38)
(38)
Using a table of Ka's, determine which of the following is the strongest acid.
(Multiple Choice)
4.8/5  (37)
(37)
Use the following acid-dissociation equilibrium constants for
 -Solutions of each acid are prepared in which the initial concentration of the acid is 0.1000 M. Which of the four solutions will be the most acidic?
-Solutions of each acid are prepared in which the initial concentration of the acid is 0.1000 M. Which of the four solutions will be the most acidic?
(Multiple Choice)
4.8/5  (38)
(38)
Which of the following would form the most basic 0.100 M solution?
(Multiple Choice)
4.8/5  (29)
(29)
"Cocaine", as it is shown on television, is actually a salt known as cocaine hydrochloride, [C17H22NO4+][Cl-], which forms white crystals that stupid people snort through straws to make them even more stupid. Recently, a lot of attention has been paid to a frightening substance known as "crack", which is formed by treating cocaine with sodium hydrogen carbonate, NaHCO3. "Crack" is a neutral compound with the formula C17H21NO4. Which of the following statements is true?
(Multiple Choice)
4.9/5  (39)
(39)
Which of the following solutions would be the best buffer capable of resisting change in pH when a small amount of NaOH is added to the solution?
(Multiple Choice)
4.8/5  (37)
(37)
Using a table of Ka's, determine which of the following is the strongest base.
(Multiple Choice)
4.8/5  (29)
(29)
What would be the effect of doubling the NH3 concentration in the solution described in the previous question?
(Multiple Choice)
4.8/5  (39)
(39)
Which of the following isn't true for the H3O+ ion concentration in a
0)0100 M solution of acetic acid (Ka = 1.8 x 10-5)?
(Multiple Choice)
4.8/5  (40)
(40)
Showing 81 - 100 of 125
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)