Exam 6: Metabolism: Energy and Enzymes
Exam 1: A View of Life49 Questions
Exam 2: Basic Chemistry57 Questions
Exam 3: The Chemistry of Organic Molecules48 Questions
Exam 4: Cell Structure and Function54 Questions
Exam 5: Membrane Structure and Function50 Questions
Exam 6: Metabolism: Energy and Enzymes55 Questions
Exam 7: Photosynthesis42 Questions
Exam 8: Cellular Respiration48 Questions
Exam 9: The Cell Cycle and Cellular Reproduction54 Questions
Exam 10: Meiosis and Sexual Reproduction54 Questions
Exam 11: Mendelian Patterns of Inheritance58 Questions
Exam 12: Molecular Biology of the Gene42 Questions
Exam 13: Regulation of Gene Expression48 Questions
Exam 14: Biotechnology and Genomics48 Questions
Exam 15: Darwin and Evolution53 Questions
Exam 16: How Populations Evolve45 Questions
Exam 17: Speciation and Macroevolution53 Questions
Exam 18: Origin and History of Life54 Questions
Exam 19: Taxonomy,systematics,and Phylogeny52 Questions
Exam 20: Viruses,bacteria,and Archaea41 Questions
Exam 21: Protist Evolution and Diversity42 Questions
Exam 22: Fungi Evolution and Diversity52 Questions
Exam 23: Plant Evolution and Diversity51 Questions
Exam 24: Flowering Plants: Structure and Organization55 Questions
Exam 25: Flowering Plants: Nutrition and Transport52 Questions
Exam 26: Flowering Plants: Control of Growth Responses54 Questions
Exam 27: Flowering Plants: Reproduction44 Questions
Exam 28: Invertebrate Evolution51 Questions
Exam 29: Vertebrate Evolution51 Questions
Exam 30: Human Evolution48 Questions
Exam 31: Animal Organization and Homeostasis48 Questions
Exam 32: Circulation and Cardiovascular Systems51 Questions
Exam 33: The Lymphatic and Immune Systems53 Questions
Exam 34: Digestive Systems and Nutrition52 Questions
Exam 35: Respiratory Systems45 Questions
Exam 36: Body Fluid Regulation and Excretory Systems47 Questions
Exam 37: Neurons and Nervous Systems49 Questions
Exam 38: Sense Organs50 Questions
Exam 39: Locomotion and Support Systems48 Questions
Exam 40: Hormones and Endocrine Systems47 Questions
Exam 41: Reproductive Systems51 Questions
Exam 42: Animal Development49 Questions
Exam 43: Behavioral Ecology48 Questions
Exam 44: Population Ecology47 Questions
Exam 45: Community and Ecosystem Ecology51 Questions
Exam 46: Major Ecosystems of the Biosphere54 Questions
Exam 47: Conservation of Biodiversity47 Questions
Select questions type
Which of these statements is NOT a consequence of the second law of thermodynamics?
(Multiple Choice)
4.7/5  (39)
(39)
Study the graph at right.The letter 'B ' depicts the _____,while 'C' depicts ________. 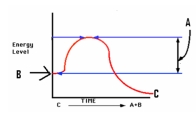
(Multiple Choice)
4.8/5  (31)
(31)
Which statement describes the currently accepted theory of how an enzyme and its substrate fit together?
(Multiple Choice)
4.8/5  (41)
(41)
During the conversion of glucose into a free form of energy only a small percentage is converted into useable ATP.What is the rest of the energy converted into?
(Multiple Choice)
4.8/5  (40)
(40)
In this pathway,B C is coupled with ADP ATP.Categorize the reactions as endergonic or exergonic. 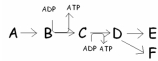
(Multiple Choice)
4.7/5  (30)
(30)
An enzyme is a globular protein that inhibits the formation of chemical bonds within the enzyme's substrate(s)causing the rate of the reaction to slow down.
(True/False)
4.8/5  (34)
(34)
While we are sitting down to lunch we are consuming _______ energy which will then be converted into ______ energy as we work until dinner time.
(Multiple Choice)
4.7/5  (37)
(37)
The energy for ATP synthesis in chemiosmotic phosphorylation comes from the movement of hydrogen ions across a membrane down a concentration gradient.
(True/False)
4.9/5  (39)
(39)
In the electron transport systems of chloroplasts and mitochondria,
(Multiple Choice)
4.9/5  (37)
(37)
Which of the following is consistent with the laws of physics governing energy?
(Multiple Choice)
4.7/5  (33)
(33)
Which statement is NOT true about how various conditions will effect the activity of an enzyme?
(Multiple Choice)
4.9/5  (37)
(37)
Coupling occurs when the energy released by an exergonic reaction is:
(Multiple Choice)
4.9/5  (32)
(32)
If a change in pH alters an allosteric site where an inhibitor binds,but doesn't change the active site for the intended substrate,it would be possible for an enzymatically controlled reaction to occur as normal.
(True/False)
4.9/5  (38)
(38)
Astrophysicists explain that eventually the sun will swell to become a red giant,engulf the earth and "burn out" with all forms of energy dispersing in a final "heat death." Compared with conditions today,the entropy of the universe then will
(Multiple Choice)
4.9/5  (28)
(28)
ATP is considered a high-energy compound because under cellular conditions,7.3 kcal per mole of energy is released when a bond is broken between:
(Multiple Choice)
4.9/5  (25)
(25)
An automobile engine is about 20 - 30% efficient in converting chemical energy to mechanical energy.Cells are about 39% efficient in the transformation of glucose to ATP.The rest of the energy is lost as heat.This is illustrative of the:
(Multiple Choice)
4.9/5  (42)
(42)
Showing 21 - 40 of 55
Filters
- Essay(0)
- Multiple Choice(0)
- Short Answer(0)
- True False(0)
- Matching(0)